The reaction 27. Identify the type of chemical reaction represented by the equation. between magnesium and an aqueous solution of silver chemical change nitrate is Has answer Review later represented by the unbalanced 28. Balance the equation using the smallest whole-number coefficients. equation below. _Mg (s) + _AGNO, (aq) →__Mg(NO,)½ (aq) + AGNO, 1 Mg(NO,)2(aq) + 1 Ag(s) Mg(s) + 1
The reaction 27. Identify the type of chemical reaction represented by the equation. between magnesium and an aqueous solution of silver chemical change nitrate is Has answer Review later represented by the unbalanced 28. Balance the equation using the smallest whole-number coefficients. equation below. _Mg (s) + _AGNO, (aq) →__Mg(NO,)½ (aq) + AGNO, 1 Mg(NO,)2(aq) + 1 Ag(s) Mg(s) + 1
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter8: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 34A
Related questions
Question
Stuck on 28
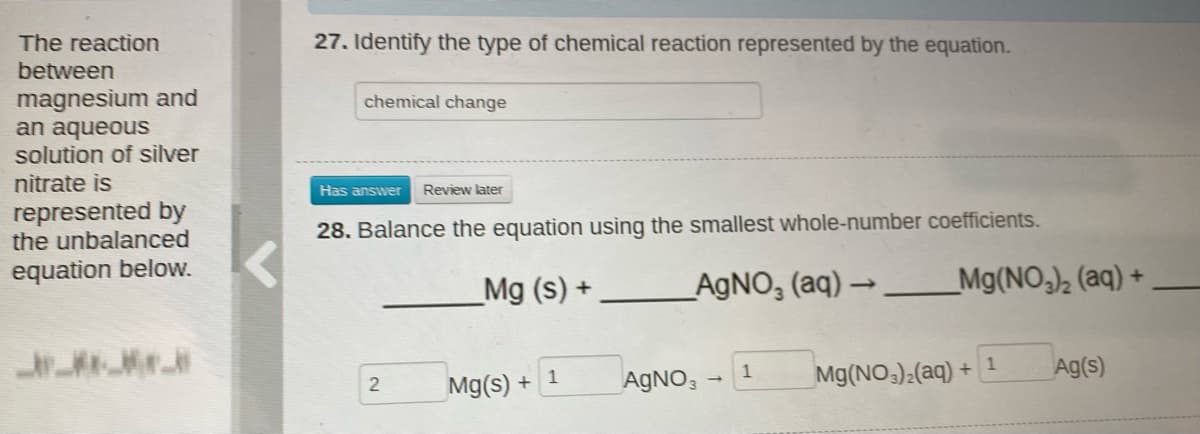
Transcribed Image Text:The reaction
27. Identify the type of chemical reaction represented by the equation.
between
magnesium and
an aqueous
solution of silver
chemical change
nitrate is
Has answer
Review later
represented by
the unbalanced
28. Balance the equation using the smallest whole-number coefficients.
equation below.
_Mg (s) +
_AGNO, (aq) → __Mg(NO,)½ (aq) +
1
Mg(NO):(aq) +
1
Ag(s)
Mg(s) + 1
AGNO,
Expert Solution
Step 1q

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning