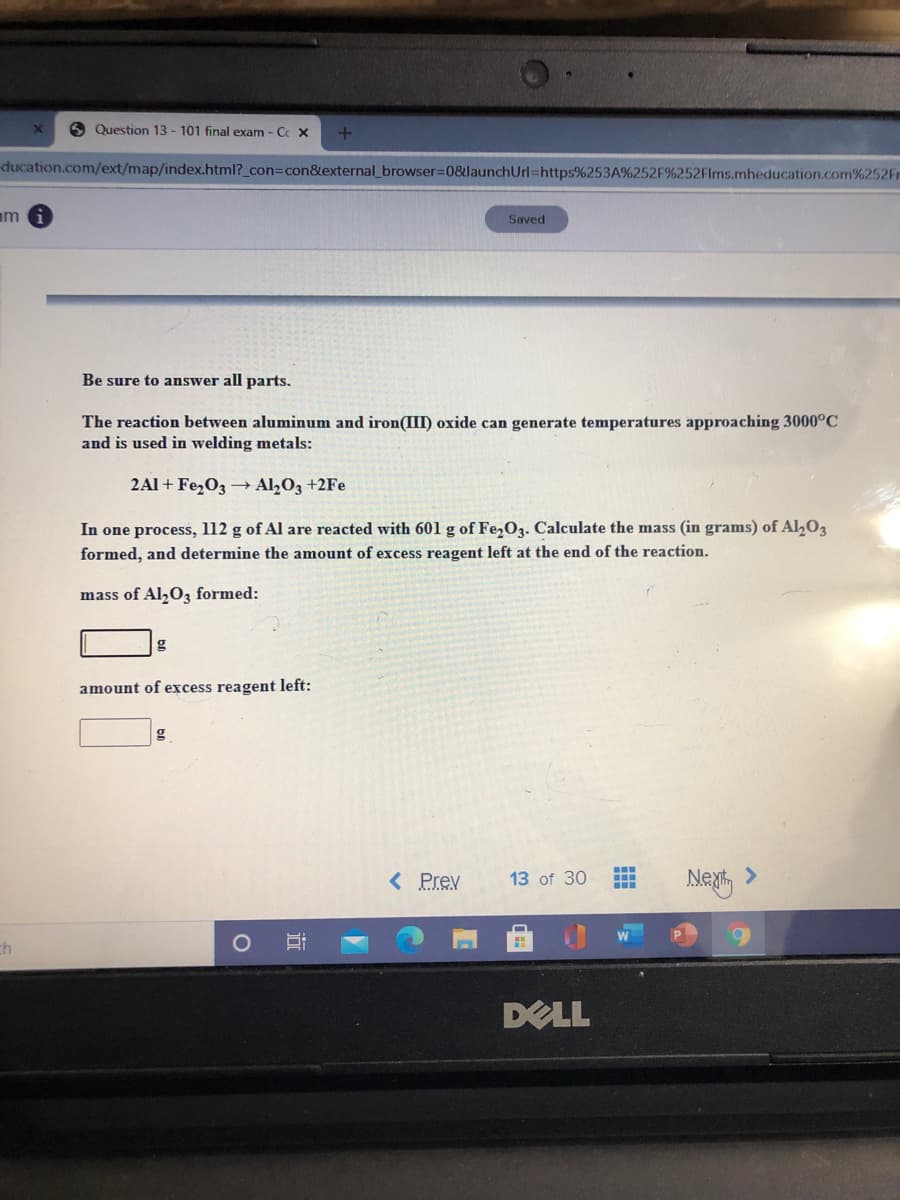
The reaction between aluminum and iron(III) oxide can generate temperatures approaching 3000°C and is used in welding metals: 2Al + Fe,O3 → A1½O3 +2Fe In one process, l12 g of Al are reacted with 601 g of Fe,O3. Calculate the mass (in grams) of Al,03 formed, and determine the amount of excess reagent left at the end of the reaction. mass of Al,0z formed: amount of excess reagent left:
The reaction between aluminum and iron(III) oxide can generate temperatures approaching 3000°C and is used in welding metals: 2Al + Fe,O3 → A1½O3 +2Fe In one process, l12 g of Al are reacted with 601 g of Fe,O3. Calculate the mass (in grams) of Al,03 formed, and determine the amount of excess reagent left at the end of the reaction. mass of Al,0z formed: amount of excess reagent left:
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 113AE: Gold is produced electrochemically from an aqueous solution of Au(CN)2 containing an excess of CN....
Related questions
Question
Transcribed Image Text:O Question 13 - 101 final exam - Cc X
ducation.com/ext/map/index.html?_con3con&external_browser=D0&launchUrl=https%253A%252F%252Flms.mheducation.com%252Fr
am
Saved
Be sure to answer all parts.
The reaction between aluminum and iron(III) oxide can generate temperatures approaching 3000°C
and is used in welding metals:
2Al + FezO3 Al2O3 +2Fe
In one process, l12 g of Al are reacted with 601 g of Fe,O3. Calculate the mass (in grams) of Al,O3
formed, and determine the amount of excess reagent left at the end of the reaction.
mass of Al,0, formed:
g
amount of excess reagent left:
< Prev
13 of 30
Ma >
ch
DELL
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning