The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water What is the maximum mass of H,O that can be produced by combining 51.8 g of each reactant? 4 NH, (g)5 02(g) 4 NO (g) 6H2O(g) 8 H,О mass
The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water What is the maximum mass of H,O that can be produced by combining 51.8 g of each reactant? 4 NH, (g)5 02(g) 4 NO (g) 6H2O(g) 8 H,О mass
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.121QP
Related questions
Question
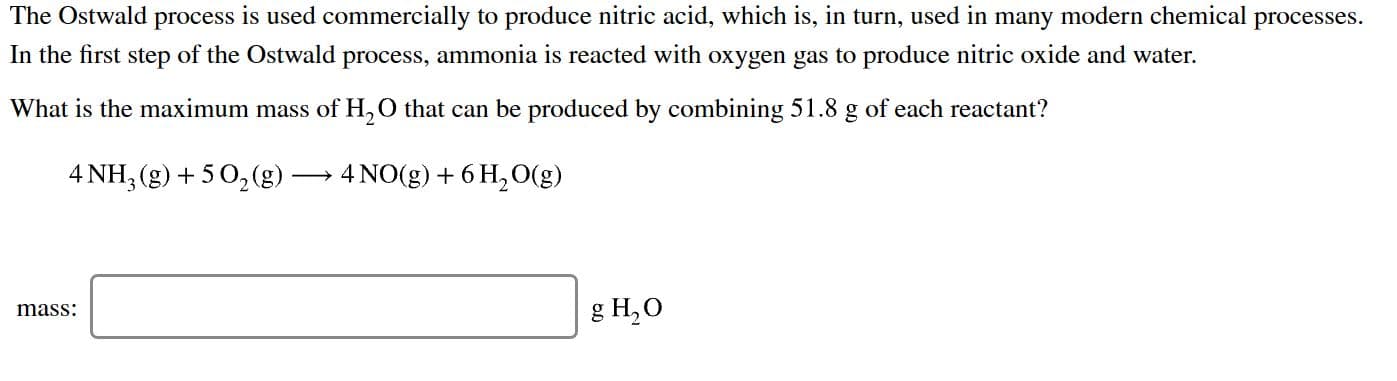
Transcribed Image Text:The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes
In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water
What is the maximum mass of H,O that can be produced by combining 51.8 g of each reactant?
4 NH, (g)5 02(g)
4 NO (g) 6H2O(g)
8 H,О
mass
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning