The solubility of a certain compound in ice-cold water is 0.22 g in 100 mL. Its solubility in hot water is 2.35 g in 100 mL. What is the best possible percent recovery that can expected for recrystallization of this compound from water? percent recovery: 90 %
The solubility of a certain compound in ice-cold water is 0.22 g in 100 mL. Its solubility in hot water is 2.35 g in 100 mL. What is the best possible percent recovery that can expected for recrystallization of this compound from water? percent recovery: 90 %
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter17: Aldehydes And Ketones
Section: Chapter Questions
Problem 17.26P: 17-26 Account for the fact that acetone has a higher boiling point (56°C) than ethyl methyl ether...
Related questions
Question
Please answer these 2

Transcribed Image Text:The solubility of a certain compound in ice-cold water is 0.22 g in 100 mL. Its solubility in hot water is 2.35 g in 100 mL.
What is the best possible percent recovery that can expected for recrystallization of this compound from water?
percent recovery: 90
%
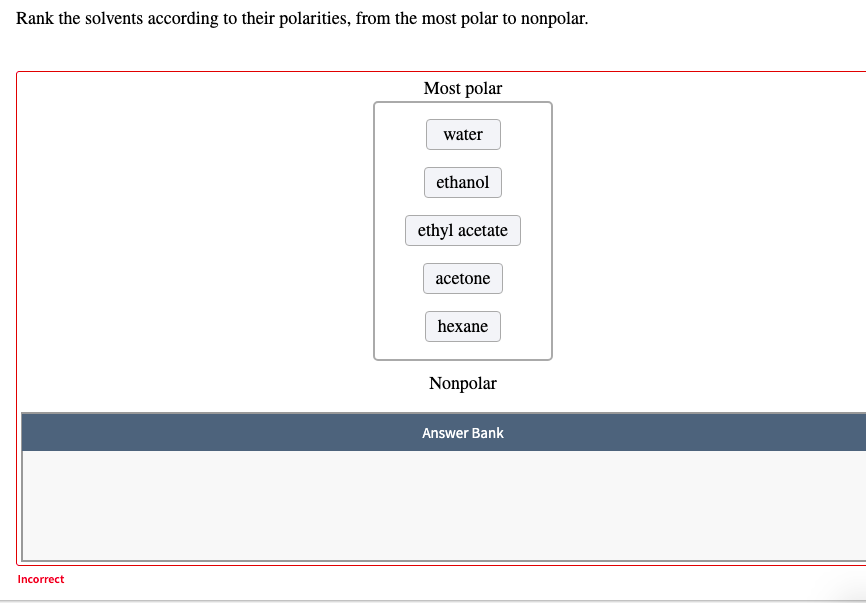
Transcribed Image Text:Rank the solvents according to their polarities, from the most polar to nonpolar.
Most polar
water
ethanol
ethyl acetate
acetone
hexane
Nonpolar
Answer Bank
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning