the statement is FALSE. If the statement is false, e BOLD word or words to make the statement true. ubstances formed as a result of a chemical reaction are АСТANTS. EMICAL EQUATION uses symbols and formulas to a reaction. nber written in front of a chemical symbol or formula COEFFICIENT. lance the following equation, the number 2 should be front of 02: KCIO3 ---------à KCI 02 YNTHESIS reaction, complex substances from ubstances. ormation of carbon dioxide during combustion of fuel nple of a DECOMPOSITION n. exothermic reaction, products have MORE energy than
the statement is FALSE. If the statement is false, e BOLD word or words to make the statement true. ubstances formed as a result of a chemical reaction are АСТANTS. EMICAL EQUATION uses symbols and formulas to a reaction. nber written in front of a chemical symbol or formula COEFFICIENT. lance the following equation, the number 2 should be front of 02: KCIO3 ---------à KCI 02 YNTHESIS reaction, complex substances from ubstances. ormation of carbon dioxide during combustion of fuel nple of a DECOMPOSITION n. exothermic reaction, products have MORE energy than
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 139A
Related questions
Question
classify the following chemical reaction 1 through 5
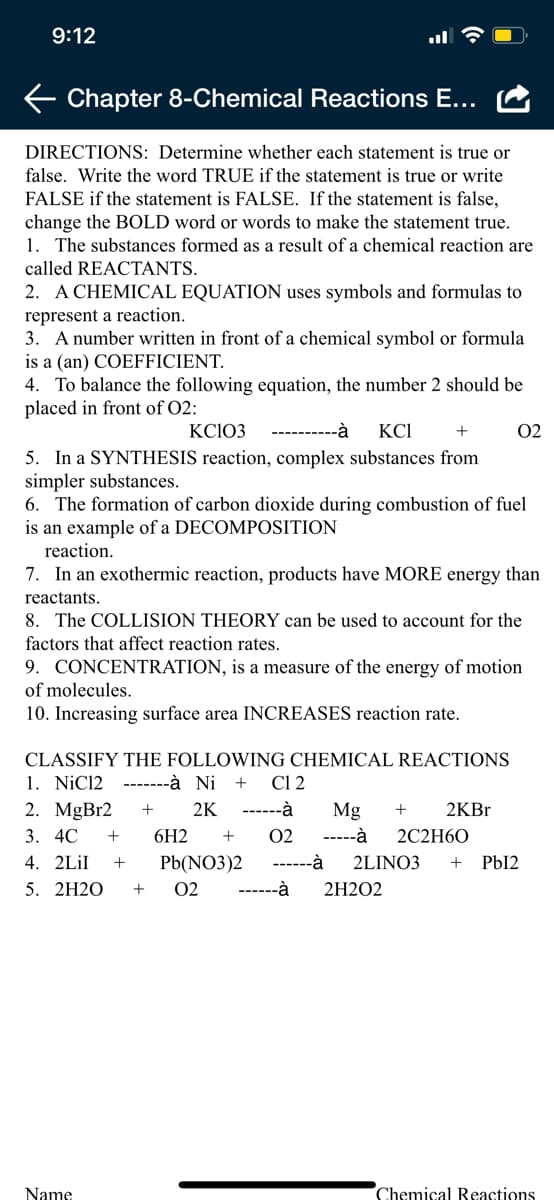
Transcribed Image Text:9:12
Chapter 8-Chemical Reactions E...
DIRECTIONS: Determine whether each statement is true or
false. Write the word TRUE if the statement is true or write
FALSE if the statement is FALSE. If the statement is false,
change the BOLD word or words to make the statement true.
1. The substances formed as a result of a chemical reaction are
called REACTANTS.
2. A CHEMICAL EQUATION uses symbols and formulas to
represent a reaction.
3. A number written in front of a chemical symbol or formula
is a (an) COEFFICIENT.
4. To balance the following equation, the number 2 should be
placed in front of O2:
KC103
----------à
KCI
02
5. In a SYNTHESIS reaction, complex substances from
simpler substances.
6. The formation of carbon dioxide during combustion of fuel
is an example of a DECOMPOSITION
reaction.
7. In an exothermic reaction, products have MORE energy than
reactants.
8. The COLLISION THEORY can be used to account for the
factors that affect reaction rates.
9. CONCENTRATION, is a measure of the energy of motion
of molecules.
10. Increasing surface area INCREASES reaction rate.
CLASSIFY THE FOLLOWING CHEMICAL REACTIONS
1. NiC12 -------à Ni +
2. MgBr2
Cl 2
2K
------à
Mg
2KB.
3. 4C
6H2
02
-----à
2C2H60
4. 2LİI
Pb(NO3)2
------à
2LINO3
+
PbI2
5. 2H2O
02
------à
2H2O2
+
Name
Chemical Beactions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
