The temperature of a sample of silver increased by 23.4 °C when 273 J of heat was applied. Substance Specific heat J/(g • °C) lead 0.128 What is the mass of the sample? silver 0.235 сорper 0.385 iron 0.449 aluminum 0.903 m = g
The temperature of a sample of silver increased by 23.4 °C when 273 J of heat was applied. Substance Specific heat J/(g • °C) lead 0.128 What is the mass of the sample? silver 0.235 сорper 0.385 iron 0.449 aluminum 0.903 m = g
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.95PAE: 9.95 How much heat is required to convert 250 g of water from liquid at 23°C: into steam at 107°C?...
Related questions
Question
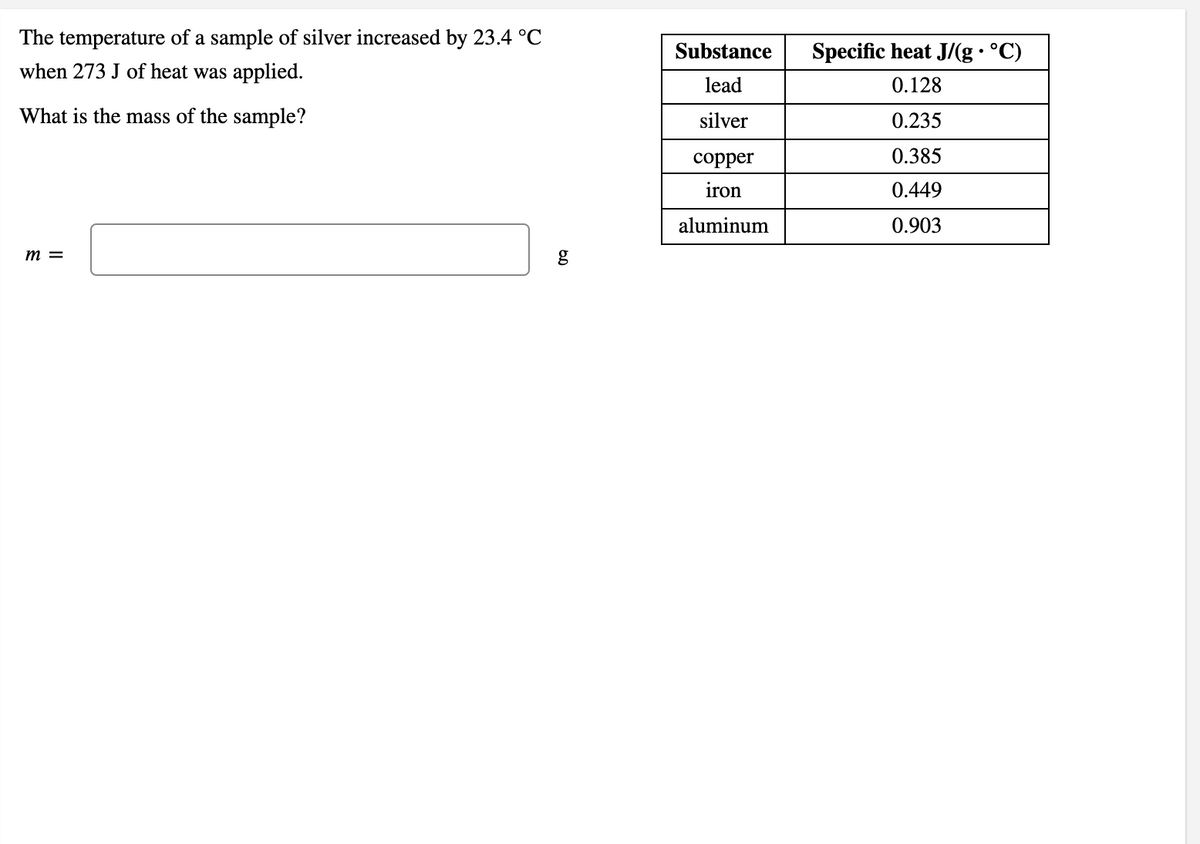
Transcribed Image Text:The temperature of a sample of silver increased by 23.4 °C
when 273 J of heat was applied.
Substance
Specific heat J/(g • °C)
lead
0.128
What is the mass of the sample?
silver
0.235
сорper
0.385
iron
0.449
aluminum
0.903
m =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning