The third paragraph should include a detailed explanation of the sources of error in Kinetics of Solvolysis of 2-chloro-2-methylpropane experiment. What experimental parameters will cause the rate constant to be larger or smaller? What are the limiting factors in the precision of the rate constant determination? What changes could you make to determine the rate constant for this reaction with better precision?
The third paragraph should include a detailed explanation of the sources of error in Kinetics of Solvolysis of 2-chloro-2-methylpropane experiment. What experimental parameters will cause the rate constant to be larger or smaller? What are the limiting factors in the precision of the rate constant determination? What changes could you make to determine the rate constant for this reaction with better precision?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section11.3: Rate Law And Order Of Reactions
Problem 11.5PSP
Related questions
Question
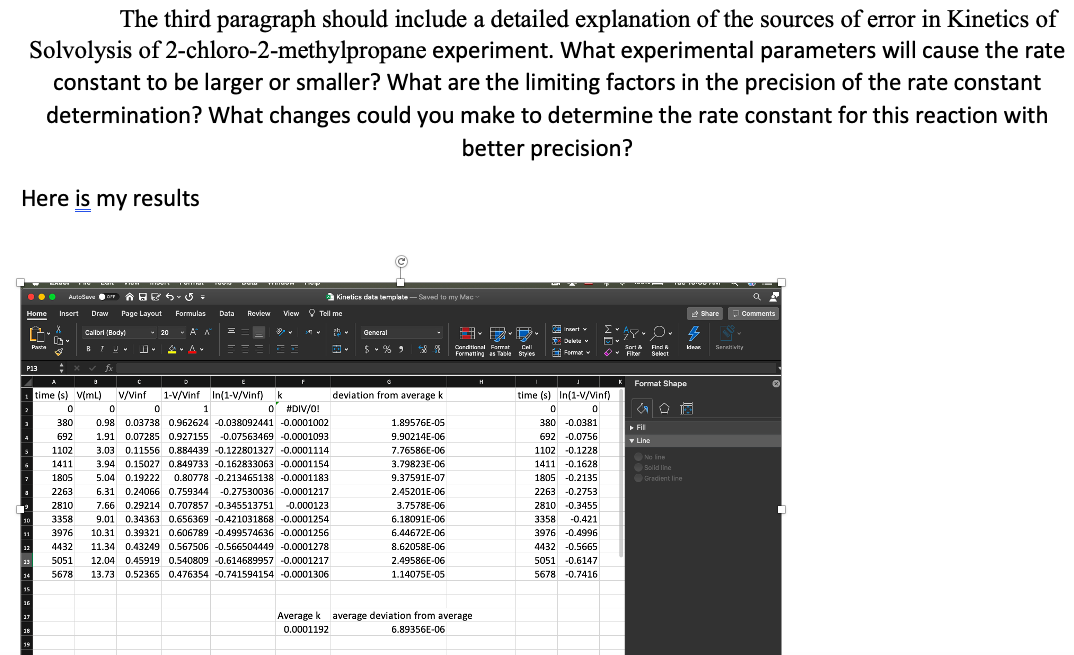
I attached my results for the graph.
Transcribed Image Text:The third paragraph should include a detailed explanation of the sources of error in Kinetics of
Solvolysis of 2-chloro-2-methylpropane experiment. What experimental parameters will cause the rate
constant to be larger or smaller? What are the limiting factors in the precision of the rate constant
determination? What changes could you make to determine the rate constant for this reaction with
better precision?
Here is my results
www
Kinetics dats template- Saved to my Mac
Auloseve
Home
Insert Draw
Page Lavout
Formulas
Data
Review
View V Tell me
A Share
-Comments
山,,。
“知。D. f S,
K nt v
Σ
Callbri (Body)
- 20
General
Dekie v
D四。
Cell
Formatting as Tasle ty es
Pata
BIJ V
Canditianal Farmat
i Femar
Sort a
Find A
Seralt vity
Fiber
Seleat
P13
fx
K Format Shape
time (s) V(ml)
V/Vinf
1-V/Vinf In(1-V/Vinf)
k
deviation from average k
time (s) In(1-V/Vinf)
o #DIV/O!
380
0.98 0.03738 0.962624 -0.038092441 -0.0001002
1.89576E-05
380 -0.0381
Fil
692
1.91 0.07285 0.927155 -0.07563469 -0.0001093
9.90214E-06
692 -0.0756
v Line
1102
3.03 0.11556 0.884439 -0.122801327 -0.0001114
7.76586E-06
1102 -0.1228
O Ne ine
Sold line
1411
3.94 0.15027 0.849733 -0.162833063 -0.0001154
3.79823E-06
1411 -0.1628
1805
5.04 0.19222
0.80778 -0.213465138 -0.0001183
9.37591E-07
1805 -0.2135
O
Gradient line
2263
6.31 0.24066 0.759344
-0.27530036 -0.0001217
2.45201E-06
2263 -0.2753
2810
7.66 0.29214 0.707857 -0.345513751 -0.000123
3.7578E-06
2810
-0.3455
3358
9.01
0.34363 0.656369 -0.421031868 -0.0001254
6.18091E-06
3358
-0.421
10
3976
10.31 0.39321 0.606789 -0.499574636 -0.0001256
6.44672E-06
3976 -0.4996
1
12
4432
11.34 0.43249 0.567506 -0.566504449 -0.0001278
8.62058E-06
4432 -0.5665
5051
12.04 0.45919 0.540809 -0.614689957 -0.0001217
2.49586E-06
5051 -0.6147
13
5678
13.73 0.52365 0.476354 -0.741594154 -0.0001306
1.14075E-05
5678 -0.7416
14
15
16
Average k average deviation from average
18
0.0001192
6.89356E-06
19
Transcribed Image Text:AutoSave
a Kinetics data template
OFF
Home
Insert
Page Layout
Formulas
Data
Review
View
Tell me
2 Share
O Comments
Calibri (Body)
v 14
A A
ab v
A Insert v
General
E Delete v
= = =
$ • % 9 | 8 8
Cell
Formatting as Table Styles
Paste
s v A v
Sensitivity
.00
Conditional Format
Sort &
Filter
Find &
Select
Ideas
BIUV
H Format v
fx
Format Shape
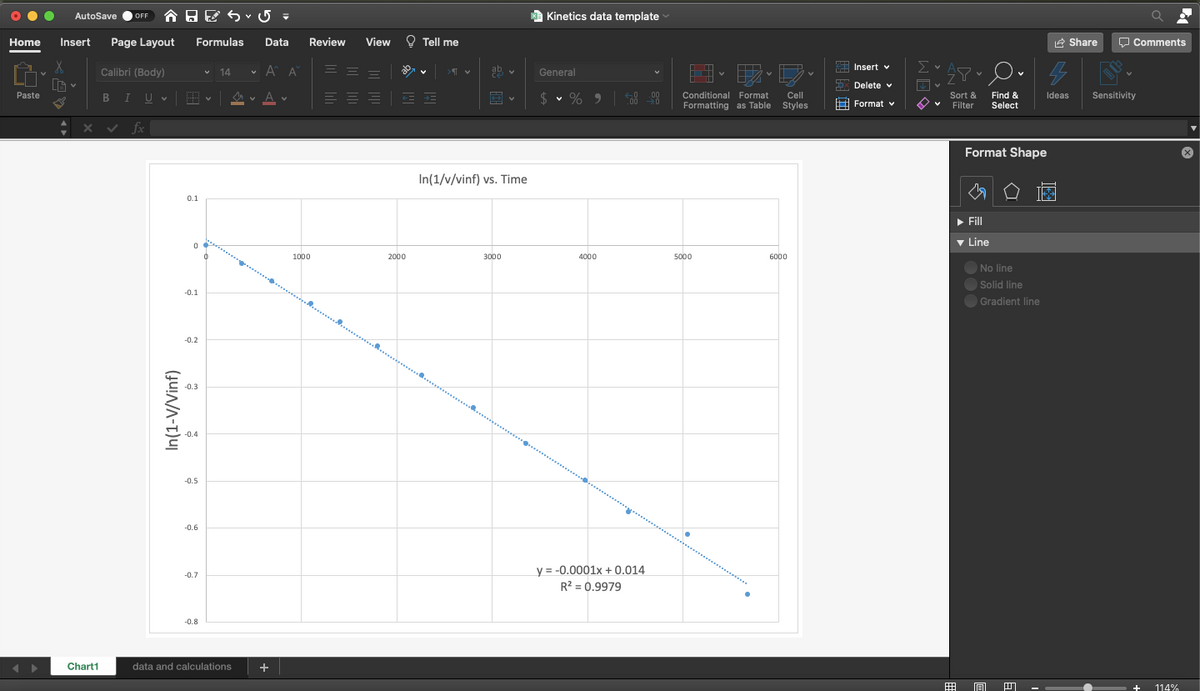
In(1/v/vinf) vs. Time
0.1
> Fill
v Line
i .. ....... ........
1000
2000
3000
4000
5000
6000
No line
Solid line
-0.1
Gradient line
-0.2
-0.3
-0.4
-0.5
-0.6
y = -0.0001x + 0.014
R? = 0.9979
-0.7
-0.8
Chart1
data and calculations
114%
In(1-V/Vinf)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,