The total pressure of the reaction mixture changes with time, as shown by the following data at 147.2°C: Time (min) Ptot (atm) Time (min) Ptot (atm) 0.2362 26 0.3322 0.2466 30 0.3449 6 0.2613 34 0.3570 10 0.2770 38 0.3687 14 0.2911 40 0.3749 18 0.3051 42 0.3801 20 0.3122 46 0.3909 22 0.3188 (a) Calculate the partial pressure of DTBP at each time from these data. Assume that at time 0, DTBP is the only gas present. (b) Are the data better described by a first-order or a second-order rate expression with respect to DTBP concentration?
The total pressure of the reaction mixture changes with time, as shown by the following data at 147.2°C: Time (min) Ptot (atm) Time (min) Ptot (atm) 0.2362 26 0.3322 0.2466 30 0.3449 6 0.2613 34 0.3570 10 0.2770 38 0.3687 14 0.2911 40 0.3749 18 0.3051 42 0.3801 20 0.3122 46 0.3909 22 0.3188 (a) Calculate the partial pressure of DTBP at each time from these data. Assume that at time 0, DTBP is the only gas present. (b) Are the data better described by a first-order or a second-order rate expression with respect to DTBP concentration?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.59E
Related questions
Question
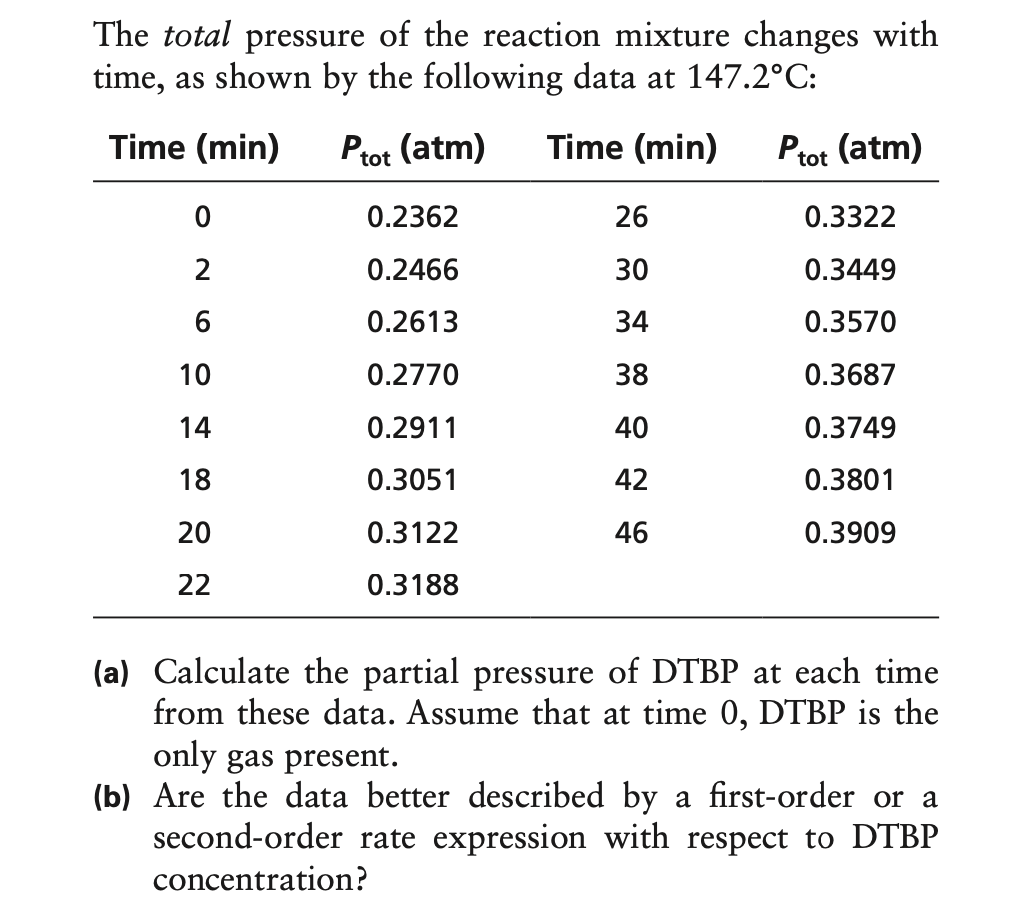
Transcribed Image Text:The total
pressure
of the reaction mixture changes with
time, as shown by the following data at 147.2°C:
Time (min)
Ptot (atm)
Time (min)
Ptot (atm)
0.2362
26
0.3322
2
0.2466
30
0.3449
0.2613
34
0.3570
10
0.2770
38
0.3687
14
0.2911
40
0.3749
18
0.3051
42
0.3801
20
0.3122
46
0.3909
22
0.3188
(a) Calculate the partial pressure of DTBP at each time
from these data. Assume that at time 0, DTBP is the
only gas present.
(b) Are the data better described by a first-order or a
second-order rate expression with respect to DTBP
concentration?
![*54. A compound called di-t-butyl peroxide [abbreviation DTBP,
formula (CH3);COOC(CH3)3] decomposes to give acetone
[(CH3),CO] and ethane (C,H,):
(CH3); COOC(CH3)3 (g)
→ 2 (CH;)2CO(g) + C2H6 (g)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8b661251-6f9a-4090-a1f5-56e54f1837ca%2Fb6bf6e26-c01d-4b2d-99f8-4ec1affab10f%2F88ozboc_processed.png&w=3840&q=75)
Transcribed Image Text:*54. A compound called di-t-butyl peroxide [abbreviation DTBP,
formula (CH3);COOC(CH3)3] decomposes to give acetone
[(CH3),CO] and ethane (C,H,):
(CH3); COOC(CH3)3 (g)
→ 2 (CH;)2CO(g) + C2H6 (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning