The water-gas reaction is a source of hydrogen. Passing steam over hot carbon produces a mixture of carbon monoxide and hydrogen. H,0(g) -C(s) (8) H (3)00 The value of K for the reaction at 1000.0°C is 3.00 x 102.
The water-gas reaction is a source of hydrogen. Passing steam over hot carbon produces a mixture of carbon monoxide and hydrogen. H,0(g) -C(s) (8) H (3)00 The value of K for the reaction at 1000.0°C is 3.00 x 102.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question
100%
Solve all parts otherwise I will downvote
Transcribed Image Text:O See p
> Question
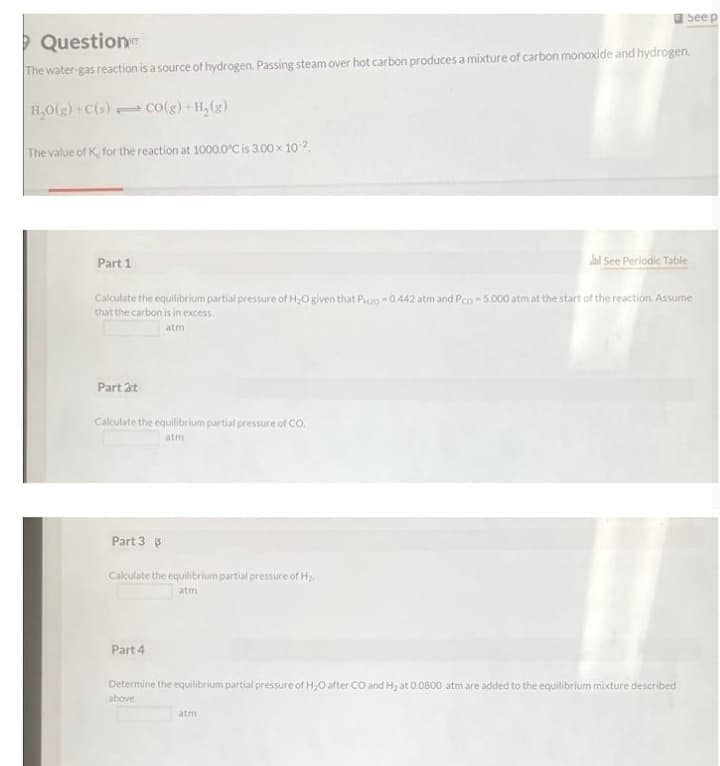
The water-gas reaction is a source of hydrogen. Passing steam over hot carbon produces a mixture of carbon monoxide and hydrogen.
H,0(g) C(s) co(g)+H,(g)
The value of K, for the reaction at 1000.0°C is 3.00 x 10 2
Part 1
lai See Periodic Table
Calculate the equilibrium partial pressure of H,O given that Poo -0.442 atm and Pco- 5.000 atm at the start of the reaction. Assume
that the carbon is in excess.
atm
Part 2t
Calculate the equilibrium partial pressure of CO.
Part 3 3
Calculate the equilibrium partial pressure of H
atm
Part 4
Determine the equilibrium partial pressure of H,O after CO and H2 at 0.0800 atmare added to the equilibrium mixture described
above
atm
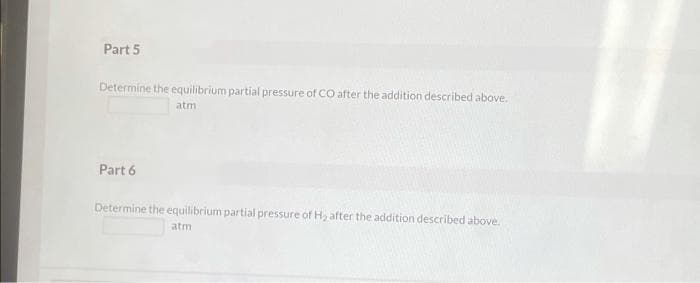
Transcribed Image Text:Part 5
Determine the equilibrium partial pressure of CO after the addition described above.
atm
Part 6
Determine the equilibrium partial pressure of H, after the addition described above.
atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning