Porosity is a measure of the void or empty spaces in a material and is a fraction of the volume of voids over the total volume. The density of the solid material used to create 13 pieces of 0.6 cm diameter and 0.67 cm height cylindrical beads is determined to be 1.53 g/mL. These beads were then placed in a flask and was filled to the 42 mL mark with a salt solution. The total weight measured of the beads and the salt solution was 51.68 g. Upon further inspection, it was found that manufacturing defects caused the measured porosity of the beads to be at 21%. Assuming that the solution was able to fully fill the empty spaces inside the beads, calculate the density of the salt solution. Show your solutions in the next item Round your answer to 2 decimal places. Add your answer
Porosity is a measure of the void or empty spaces in a material and is a fraction of the volume of voids over the total volume. The density of the solid material used to create 13 pieces of 0.6 cm diameter and 0.67 cm height cylindrical beads is determined to be 1.53 g/mL. These beads were then placed in a flask and was filled to the 42 mL mark with a salt solution. The total weight measured of the beads and the salt solution was 51.68 g. Upon further inspection, it was found that manufacturing defects caused the measured porosity of the beads to be at 21%. Assuming that the solution was able to fully fill the empty spaces inside the beads, calculate the density of the salt solution. Show your solutions in the next item Round your answer to 2 decimal places. Add your answer
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter6: Properties Of Hydrates
Section: Chapter Questions
Problem 1ASA: A student is given a sample of a pink manganese (II) chloride hydrate. She weighs the sample in a...
Related questions
Question
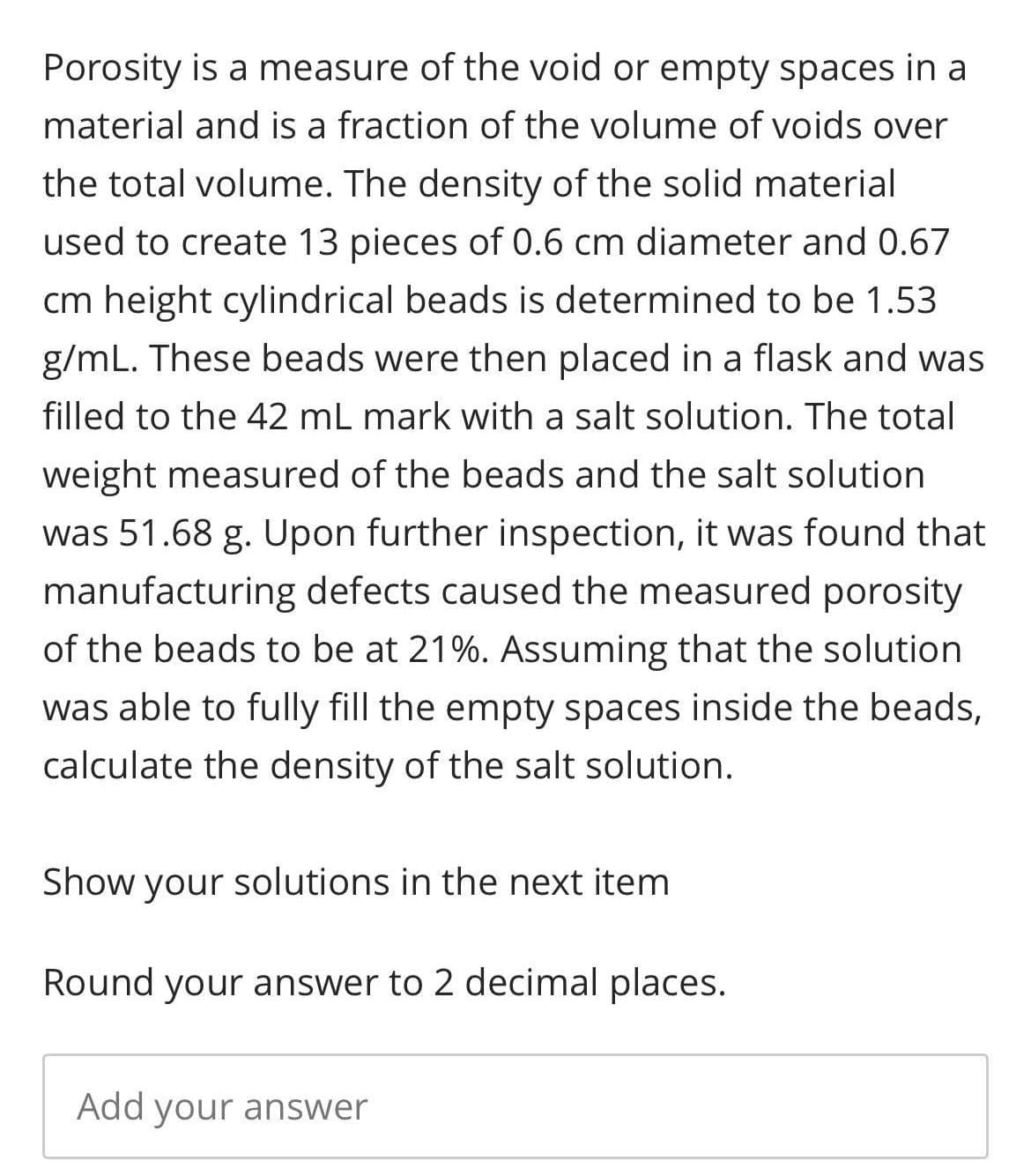
Transcribed Image Text:Porosity is a measure of the void or empty spaces in a
material and is a fraction of the volume of voids over
the total volume. The density of the solid material
used to create 13 pieces of 0.6 cm diameter and 0.67
cm height cylindrical beads is determined to be 1.53
g/mL. These beads were then placed in a flask and was
filled to the 42 mL mark with a salt solution. The total
weight measured of the beads and the salt solution
was 51.68 g. Upon further inspection, it was found that
manufacturing defects caused the measured porosity
of the beads to be at 21%. Assuming that the solution
was able to fully fill the empty spaces inside the beads,
calculate the density of the salt solution.
Show your solutions in the next item
Round your answer to 2 decimal places.
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning