thermometer A 52.7 g sample of polystyrene is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The polystyrene sample starts off at 89.5 °C and the temperature of the water starts off at 25.0 °C. When the temperature of the water stops insulated container changing it's 30.5 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of polystyrene according to this experiment. Be sure your answer is rounded to 2 significant digits. sample a calorimeter Ar J N10 g.°C X Check Explanation Privacy Terms of Use O 2019 McGraw-Hill Education. All Rights Reserved. 9:50 PM X 11/20/2019 22 Type here to search hp end S prt sc home delete fg 44 backspac & % $ 7 6 5
thermometer A 52.7 g sample of polystyrene is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The polystyrene sample starts off at 89.5 °C and the temperature of the water starts off at 25.0 °C. When the temperature of the water stops insulated container changing it's 30.5 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of polystyrene according to this experiment. Be sure your answer is rounded to 2 significant digits. sample a calorimeter Ar J N10 g.°C X Check Explanation Privacy Terms of Use O 2019 McGraw-Hill Education. All Rights Reserved. 9:50 PM X 11/20/2019 22 Type here to search hp end S prt sc home delete fg 44 backspac & % $ 7 6 5
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 65SCQ
Related questions
Question
100%
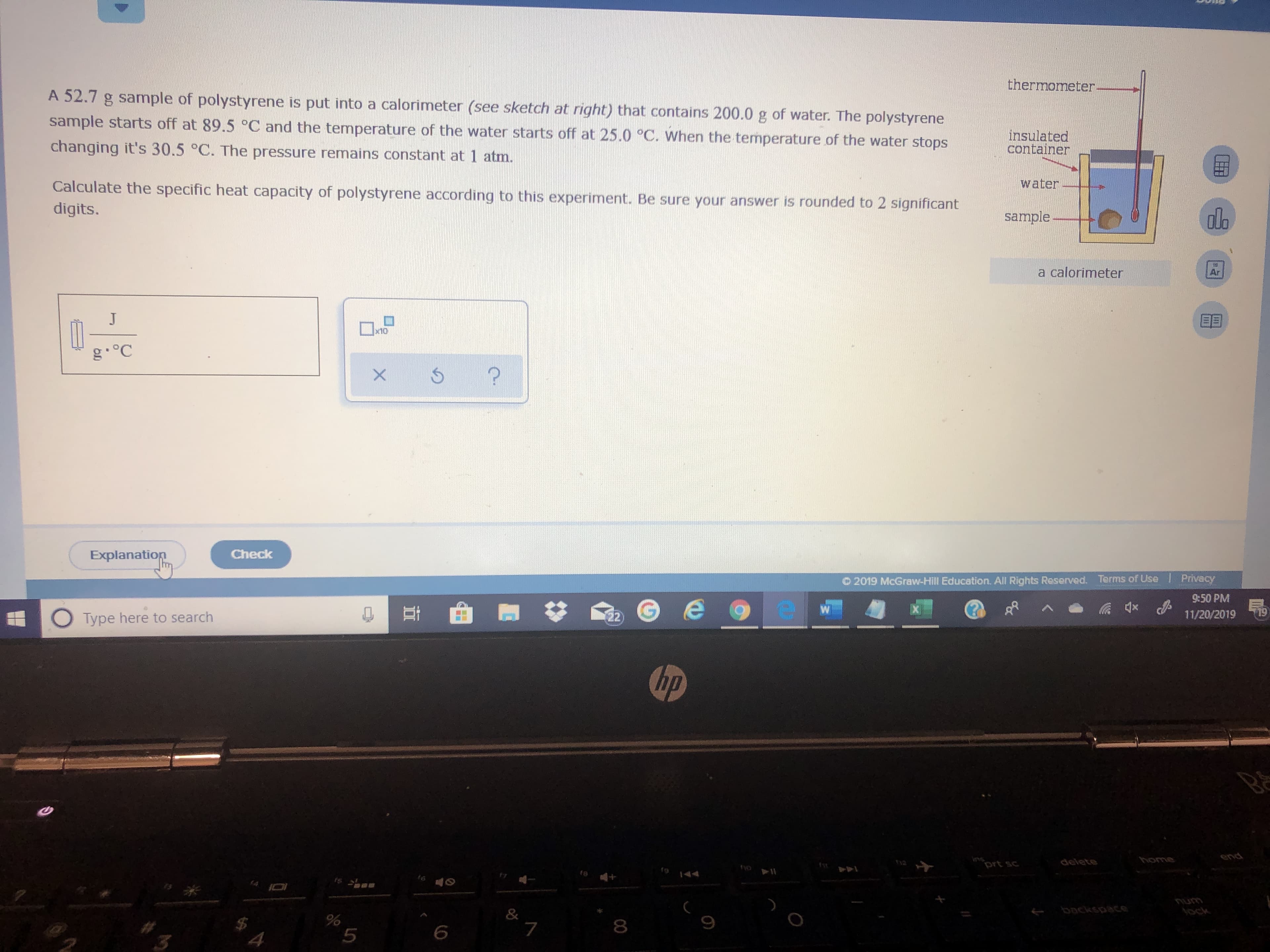
Transcribed Image Text:thermometer
A 52.7 g sample of polystyrene is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The polystyrene
sample starts off at 89.5 °C and the temperature of the water starts off at 25.0 °C. When the temperature of the water stops
insulated
container
changing it's 30.5 °C. The pressure remains constant at 1 atm.
water
Calculate the specific heat capacity of polystyrene according to this experiment. Be sure your answer is rounded to 2 significant
digits.
sample
a calorimeter
Ar
J
N10
g.°C
X
Check
Explanation
Privacy
Terms of Use
O 2019 McGraw-Hill Education. All Rights Reserved.
9:50 PM
X
11/20/2019
22
Type here to search
hp
end
S
prt sc
home
delete
fg
44
backspac
&
%
$
7
6
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning