When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution(dissolving) can be determined using a coffee cup calorimeter. In the laboratory a general chemistry student finds that when 10.37 g of BaBr2(s) are dissolved in 101.80 g of water, the temperature of the solution increases from 22.26 to 24.08 °C.
When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution(dissolving) can be determined using a coffee cup calorimeter. In the laboratory a general chemistry student finds that when 10.37 g of BaBr2(s) are dissolved in 101.80 g of water, the temperature of the solution increases from 22.26 to 24.08 °C.
Chapter10: Energy
Section: Chapter Questions
Problem 56A
Related questions
Question
| When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution(dissolving) can be determined using a coffee cup calorimeter. In the laboratory a general chemistry student finds that when 10.37 g of BaBr2(s) are dissolved in 101.80 g of water, the temperature of the solution increases from 22.26 to 24.08 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.64 J/°C. Based on the student's observation, calculate the enthalpy of dissolution of BaBr2(s) in kJ/mol. Assume the specific heat of the solution is equal to the specific heat of water. ΔHdissolution = _______ kJ/mol |
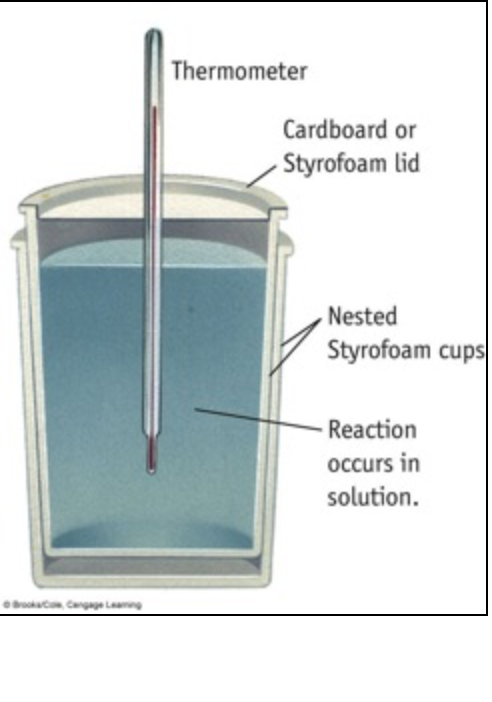
Transcribed Image Text:Thermometer
Cardboard or
Styrofoam lid
Nested
Styrofoam cups
Reaction
occurs in
solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co