Ammonium phosphate ((NH4),PO4) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH,). What mass of ammonium phosphate is produced by the reaction of 1.73 g of phosphoric acid? Be sure your answer has the correct number of significant digits. Ox10
Ammonium phosphate ((NH4),PO4) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH,). What mass of ammonium phosphate is produced by the reaction of 1.73 g of phosphoric acid? Be sure your answer has the correct number of significant digits. Ox10
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.37PAE: 3.37 If atypical grain of sand occupies a volume of 1.3 × 10-4 cm3, what is the volume (in cm3) of 1...
Related questions
Question
Ammonium phosphate ((NH4)3PO4) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H3PO4) with ammonia (NH3). What mass of ammonium phosphate is produced by the reaction of 1.73g of phosphoric acid? Be sure your answer has the correct number of significant digits.
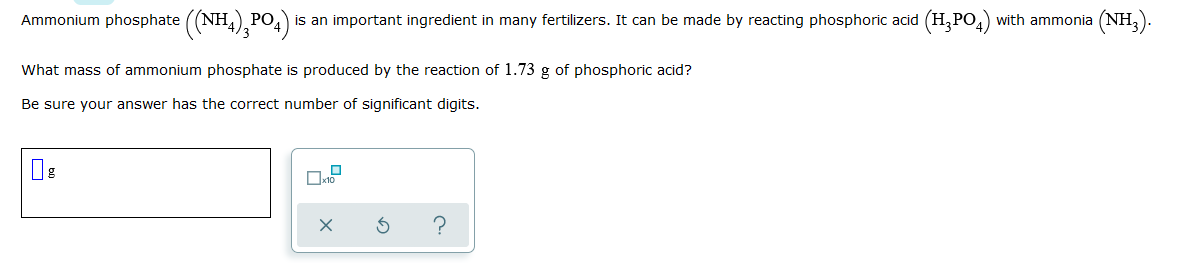
Transcribed Image Text:Ammonium phosphate ((NH4),PO4)
is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH,).
What mass of ammonium phosphate is produced by the reaction of 1.73 g of phosphoric acid?
Be sure your answer has the correct number of significant digits.
Ox10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
