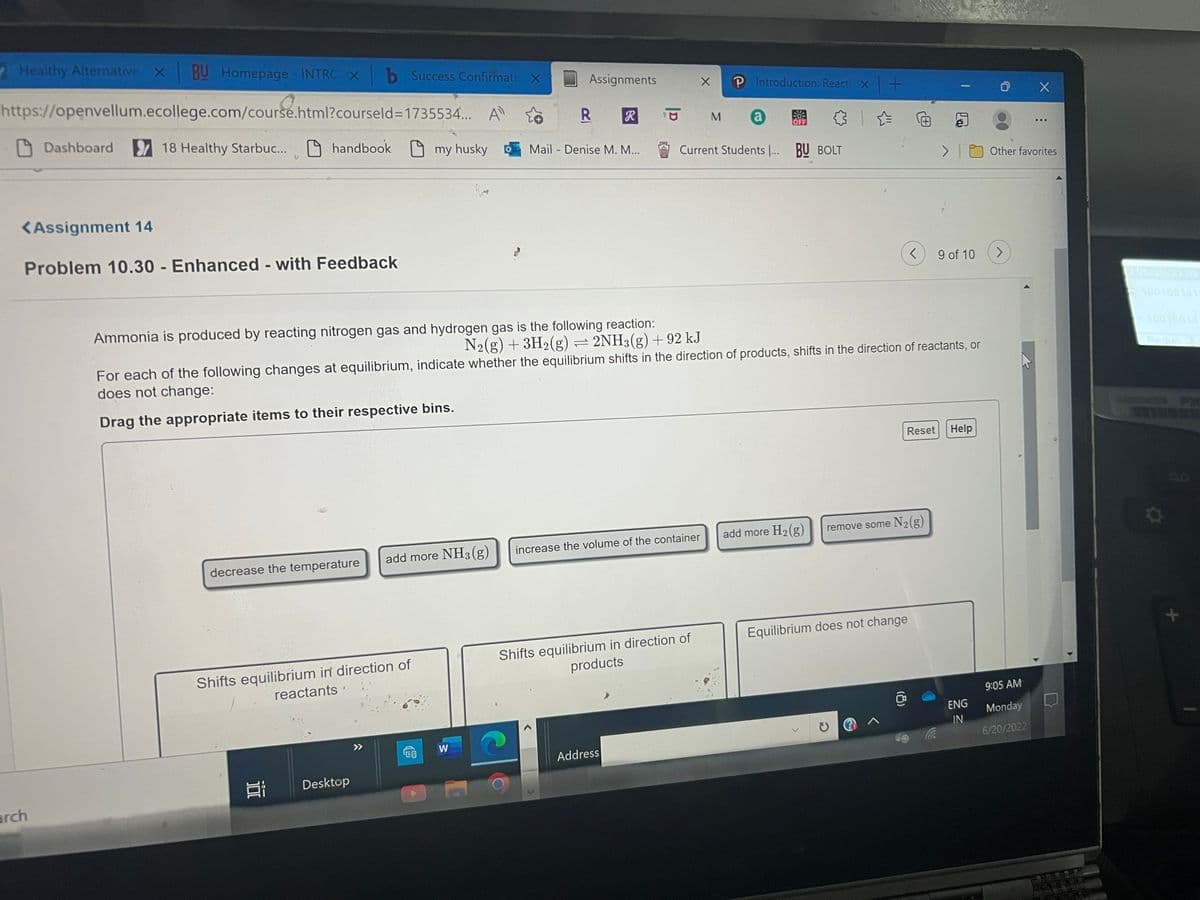
Ammonia is produced by reacting nitrogen gas and hydrogen gas is the following reaction: N2(g) + 3H₂(g) 2NH3(g) +92 kJ For each of the following changes at equilibrium, indicate whether the equilibrium shifts in the direction of products, shifts in the direction of reactants, or does not change: Drag the appropriate items to their respective bins.
Ammonia is produced by reacting nitrogen gas and hydrogen gas is the following reaction: N2(g) + 3H₂(g) 2NH3(g) +92 kJ For each of the following changes at equilibrium, indicate whether the equilibrium shifts in the direction of products, shifts in the direction of reactants, or does not change: Drag the appropriate items to their respective bins.
Chapter30: Kinetic Methods Of Analysis
Section: Chapter Questions
Problem 30.12QAP
Related questions
Question
Transcribed Image Text:Healthy Alternative X BU Homepage - INTRC x b Success Confirmatic X
Assignments
https://openvellum.ecollege.com/course.html?courseld=1735534... A sô
℗ Introduction: React x +
R
R
M
Dashboard
a
18 Healthy Starbuc...
handbook
my husky
Mail - Denise M. M...
Current Students ... BU BOLT
<Assignment 14
Problem 10.30 - Enhanced - with Feedback
< 9 of 10
Ammonia is produced by reacting nitrogen gas and hydrogen gas is the following reaction:
N₂(g) + 3H₂(g) = 2NH3(g) +92 kJ
For each of the following changes at equilibrium, indicate whether the equilibrium shifts in the direction of products, shifts in the direction of reactants, or
does not change:
Drag the appropriate items to their respective bins.
Reset
Help
add more H₂(g) remove some N₂(g)
increase the volume of the container
add more NH3(g)
decrease the temperature
Equilibrium does not change
Shifts equilibrium in direction of
products
Shifts equilibrium in direction of
reactants'
@ ^
Address
Ei Desktop
arch
1.5
W
10
X
(8)
-
...
Other favorites
J
X
9:05 AM
ENG
Monday
IN
6/20/2022
O
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
this is wrong..... here s te response... can youprovide correct please????
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

