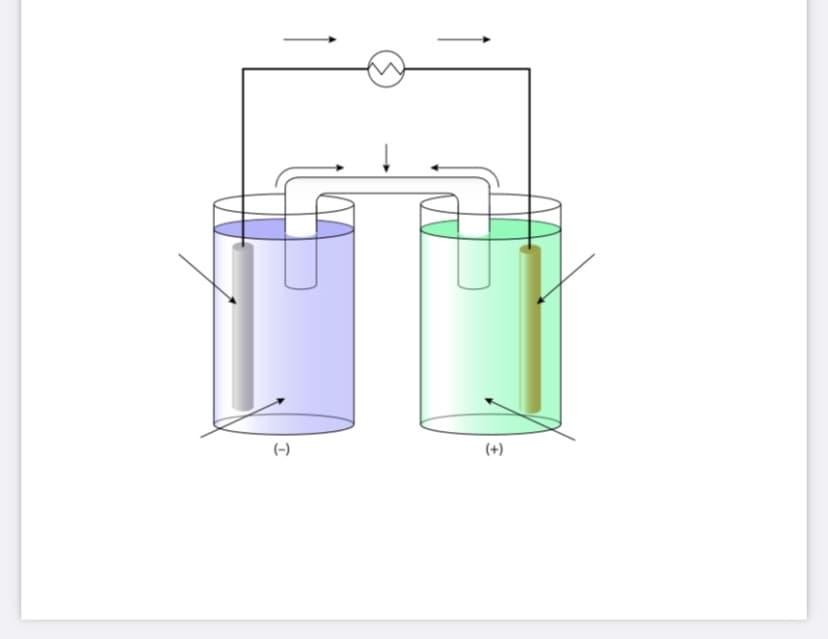
ully label the unlabelled Galvanic cell below using the following information: Electrodes: Magnesium and Zinc Salt Bridge: KNO3 Include: a. Identify the anode and cathode (and the solution each is in) b. Write the anode and cathode half-cell reactions c. Write the overall cell reaction d. Show the direction of e- flow e. Show the direction of ion flow in the salt bridge f. Predict which electrode will increase in mass and which electrode will decrease in mass. Explain your answer. Fully label the unlabelled Galvanic cell below using the following information: Electrodes: Magnesium and Zinc Salt Bridge: KNO3 Include: a. Identify the anode and cathode (and the solution each is in) b. Write the anode and cathode half-cell reactions c. Write the overall cell reaction d. Show the direction of e- flow e. Show the direction of ion flow in the salt bridge f. Predict which electrode will increase in mass and which electrode will decrease in mass. Explain your answer. Fully label the unlabelled Galvanic cell below using the following information: Electrodes: Magnesium and Zinc Salt Bridge: KNO3 Include: a. Identify the anode and cathode (and the solution each is in) b. Write the anode and cathode half-cell reactions c. Write the overall cell reaction d. Show the direction of e- flow e. Show the direction of ion flow in the salt bridge f. Predict which electrode will increase in mass and which electrode will decrease in mass. Explain your answer.
Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Step by step
Solved in 3 steps with 1 images




