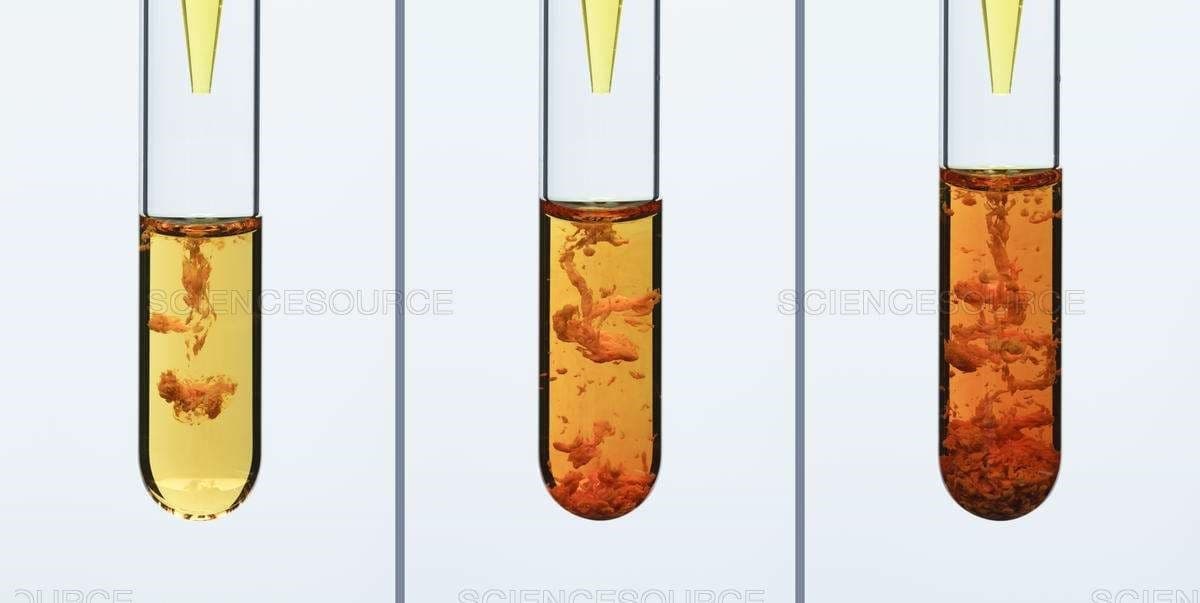
This series demonstrates addition of 0.5 M potassium chromate solution (? ??? ) drop-by-drop to 0.25 M iron(III) chloride solution (???? ). Write the net ionic equation for this reaction. What is the precipitate?
Q: What is the maximum concentration of Ni²⁺ that can be added to a 0.00910 M solution of Na₂CO₃ before…
A: Given : Concentration of Na2CO3 = 0.00910 M Since Na2CO3 is completely soluble in water. Hence it…
Q: An aqueous solution contains Ag+, Pb2+, Cu+, and Tl+ with each ion at a concentration of 0.15M. The…
A: Solubility product may be defined as the product of concentration of products each raised to the…
Q: An aqueous solution contains 0.10M concentrations of Mg", Mn", and Ni", if NaOH(aq) is added, in…
A:
Q: When 22.0 mL of a 3.58× 104M ammonium chloride solution is combined with 25.0 mL of a 9.20x104 M…
A:
Q: What is the minimum concentration of Cr3+ that must be added to 0.095 M NaF in order to initiate a…
A: Solution- In equeus solution-CrF3(s) ⇌ Cr3+(aq) +3F- (aq)Ksp=Cr3+F-3
Q: When 25.0 mL of a 7.48×104 M lead nitrate solution is combined with 25.0 mL of a 7.20×104M sodium…
A: We can use solubility product principle for a sparingly soluble substance to predict whether under…
Q: A 1.40 g sample of silver nitrate(169.97 g/mol) is dissolved in sufficient water to make 125 mL of…
A: Answer: 1.0749 g is the mass of AgCl precipitate formed.
Q: What is the effect of H2SO4 on Epsom salts? Select all that apply. No visible reaction Visible…
A: Solution MgSO4 = Magnesium sulfate Other names: Epsom salt, English salt, bitter salt, bath salt,…
Q: The substance to be analyzed – is deep-pink colored, highly soluble in water salt. Reaction with NH;…
A: With the help of given reactions and property of the substance we can identify the substance.
Q: Calculate the minimum concentration of Cr3+ that must be added to 0.095 M NaF in order to initiate a…
A: given Ksp (CH F3) = 6. 6 * 10-11 molarity of Na F = 0.095 M ∴ ( F-) = (Na F ) = 0.095 M
Q: Calcium carbonate (limestone) is a very insoluble ionic salt! Answer the following if you add a lew…
A: A question based on solutions that is to be accomplished.
Q: Cobalt(II)-chloride system 1. Prepare two test tubes (T1,T2). T1 serves as the control. 2. To both…
A: The addition of concentrated HCl to cobalt(II) ion forms a complex compound that leads to a change…
Q: Many salts of Pb2+ are sparingly soluble, and the difference in their solubility can be used to…
A:
Q: 29. 300.0 mL of 0.00325 M barium chloride is added to an equal volume of 0.00400M sodium sulfate.…
A: The precipitation reaction between Barium chloride and Sodium sulfate to form Barium Sulfate is…
Q: Which of the following metal hydroxides will precipitate third as solid KOH is added if the metal…
A: All the given ions are in same concentration. All the hydroxides formed will dissociate as one mole…
Q: It is experimentally determined that 1.33 x 103 g of silver bromide will dissolve in 100.0 mL of…
A: Solubility Product(Ksp) = It is an equilibrium constant which shows the relation between ions and…
Q: Use the References to access important values if needed for this question. When 15.0 mL of a…
A:
Q: The substance to be analyzed - is white, highly soluble in water salt. When interacting with…
A: There are several chemical tests are reported in literature for the determination of different metal…
Q: A chemical equilibrium can be established only if none of the products is allowed to escape out or…
A: Given that , A chemical equilibrium can be established only if none of the products is allowed to…
Q: A student measures the molar solubility of calcium chromate in a water solution to be 2.56×10-2 M.…
A: Solubility means maximum amount of solute that can be dissolved in per liter of solution. Solubility…
Q: Välues If needed for this question. When 12.0 mL of a 6.47×10-4 M sodium phosphate solution is…
A:
Q: Write balanced equations describing each of the reactions in Steps1 through 5. A solution may…
A: Cationic analysis refers to analysis of a salt or a mixture for cations present in them. Group I of…
Q: Use the References to access important values if needed for this question. When 15.0 mL of a…
A: Given : M1 = 4.97 ×10^-4M M2 = 5.30 ×10^-4M V1 = 15.0 ml V2 = 18.0 ml Finding : 1) Reaction…
Q: 18.0 mL of a 2.57×10-4 M iron(III) acetate solution is combined with 22.0 mL of a 6.33×10-4 M…
A: When two or more soluble salts are mixed in a solution, there is a chance of interactions of…
Q: AgCl(s), silver chloride, has a very low solubility: AgCl(s) ⇌ Ag +(aq) + Cl−(aq), Ksp = 1.6 × 10–10…
A: Calculating K value- AgCl(s) ⇌ Ag+(aq) + Cl−(aq) Ksp = 1.6 ×…
Q: hydroxide solution is combined with 22.0 mL of a 8.53×10-4 M nickel(II) fluoride solution does a…
A:
Q: Consider dissolving the barium nitrate in nitric acid HNO3, a strong acid (it reacts completely with…
A: Components in the solution are : Barium nitrate : Ba(NO3)2 Strong acid : HNO3 The question is to…
Q: When 18.0 mL of a 2.81x10 M potassium sulfide solution is combined with 18.0 mL of a 9.52x10 M…
A: Solubility product of a sparingly soluble salt is the equilibrium constant of the salt.
Q: Using the solubility guidelines. Write a complete set of equations (molecular, total ions, and net…
A:
Q: A solution is 0.0400M in both CrO42- and SO42. Slowly, Pb(NO3)2 is added to this solution. What is…
A: Given: Ksp of PbCrO4 = 2.80 × 10-13 Ksp of PbSO4 = 1.60 × 10-8 [CrO42-] = 0.0400 M. [SO42-] =…
Q: Based on the chemical equation provided, what do you think is equivalent to K1? a. [Ag(NH3)2+] /…
A: Equilibrium constant expression is represented the ratio of concentration of products to…
Q: A student measures the F- concentration in a saturated aqueous solution of barium fluoride to be…
A:
Q: Many salts of Pb2+ are sparingly soluble, and the difference in their solubility can be used to…
A:
Q: Calculate the solubility of copper (II) iodate in 0.21 M copper (II) nitrate. Ksp* is 7.4x10-8M3.
A: Since you have posted multiple questions, we are entitled to answer the first only.
Q: ample Mass of Original Mixture (g) Mass of Watch Glass (g) Mass of Filter Paper (g) Mass of…
A: The reagent that consumes completely in a chemical reaction is termed as the limiting agent. The…
Q: Why is it normal to add a slight excess of silver nitrate solution when precipitating chloride ion?
A:
Q: Suppose that equal volumes of 0.114 M sodium chloride (NaCl) and 0.0888 M silver nitrate (AgNO3) are…
A:
Q: 18.0 mL of a 6.12 x 10^-4 M chromium (III) nitrate solution is combined with 22.0 mL of a 3.53 x…
A:
Q: The substance to be analyzed – is white, highly soluble in water salt. When interacting with…
A: Salt analysis:
Q: If 25 mL of a 0.10 M solution of sodium sulfate solution is added to 40. mL of a 0.20 M lead (II)…
A: The balanced chemical equation representing the chemical reaction between sodium sulfate solution…
Q: Using a first to last order structure, explain why and write the order in which K, Au', Pb", Al",…
A: The solution of the question is given below:
Q: This series demonstrates a progression as 0.5 M solution of sodium carbonate (??2Co3) as it is added…
A:
Q: ). If Ca(NO3) (aq) is slowly added to solution containing CO32– and SO42– what compound would…
A:
Q: Scoring: Your score will be based on the number of correct matches minus the number of incorrect…
A:
Q: 9. Given that CuBr would precipitate later than Cul in a solution containing equimolar…
A: Solubility product defines the solubility of the complex in a given solution. More the solubility…
Q: Color of pHydrion strip:…
A: Recall the expression of pH and pOH as follows pH=-logH+pOH=-logOH- Again, pH + pOH=14…
Q: For PbCl2, Ksp 1.7 x 105. What will occur if 250 mL of 0.10 M %3D Pb(NO3)2 is mixed with 250 mL of…
A:
Q: A reaction solution is made up of 10.0 ml of 4.0 M acetone, 10.0 mL of 1.0 M HCI, 10.0 mL of 0.0050…
A: Here, Volume of acetone = 10mL. Concentration of acetone = 4M. Volume of HCl = 10mL. Volume of I2 =…
Q: Using the appropriate Ksp values, find the concentration of K+ ions in the solution at equilibrium…
A: Dissociation reaction of 550 mL of Cu(NO3)2 and 500 mL KOH taking place in the solution is given.
- This series demonstrates addition of 0.5 M potassium chromate solution (? ??? ) drop-by-drop to 0.25 M iron(III) chloride solution (???? ).
- Write the net ionic equation for this reaction.
- What is the precipitate?
Step by step
Solved in 2 steps with 2 images

- Compound Δ?∘f (kJ/mol)ΔGf∘ (kJ/mol) A +387.7+387.7 B +600.4+600.4 C +402.0+402.0 Use the data given here to calculate the values of Δ?∘rxnΔGrxn∘ at 25 ∘25 ∘C for the reaction described by the equation A+B↽−−⇀C A+B↽−−⇀CA+B↽−−⇀C Δ?∘rxn=ΔGrxn∘= kJkJ If Δ?∘rxnΔHrxn∘ and Δ?∘rxnΔSrxn∘ are both positive values, what drives the spontaneous reaction and in what direction at standard conditions? The spontaneous reaction is enthalpy-driven to the left. enthalpy-driven to the right. entropy-driven to the right. entropy-driven to the left.2nd part uses the answer from the screenshot to find ΔSsyst, ΔSsurr, and ΔSuniv for reaction 3.1 (includingcorrect units)Temp (K) CV (J/mol.K) Using the general formula CV = A + BT + C/T² , find values of A, B, and C that fit the given data. Temp(K) Cv(J/mol.K) 300 20.8 400 20.9 500 21.2 600 21.8 700 21.4 800 23.1 900 23.7 1000 24.3 1100 24.9
- Based on the given data, why is Qlost + Qgained is not equal to zero? Please state what could be the possible sources of error on why it is not equal to zero? Please explain briefly (I will give a high rate)What is the balanced net inotic equation for: Pb+2(aq)+SO4-2 ----> ?Ssolve asap like is ready pls pls solve fast

