Titration of Purpose: To find the molarity (M) of acetic acid in vinegar by titration with a standardized 0.50M sodium hydroxide Solution. (acetic acid = HC₂ H3 0₂ CH3 COOH) Reaction: Acetic Acid Acetic Acid in vinegar .Vinegar Contains acetic acid 1 NaOH(aq) + 1 HC₂ H₂ Oz caq 1 Na Cz Hz Oz (og) + 1 HOH (2) ?mol (H₂O) 0.50 mal IL IL Data: .a.50MNAON Volume of vinegar Volume of NaOH initial Volume of NaoH final Volume of NaOH used Molarity of vinegar Trial 20.00 mL 0.60 mL 35.45 mL 34. 85 mc Trial 2 20.00 mL 0.85 mL 34. 30 mL Trial 3 20.00 mL 0.45mL 33.80mL
Titration of Purpose: To find the molarity (M) of acetic acid in vinegar by titration with a standardized 0.50M sodium hydroxide Solution. (acetic acid = HC₂ H3 0₂ CH3 COOH) Reaction: Acetic Acid Acetic Acid in vinegar .Vinegar Contains acetic acid 1 NaOH(aq) + 1 HC₂ H₂ Oz caq 1 Na Cz Hz Oz (og) + 1 HOH (2) ?mol (H₂O) 0.50 mal IL IL Data: .a.50MNAON Volume of vinegar Volume of NaOH initial Volume of NaoH final Volume of NaOH used Molarity of vinegar Trial 20.00 mL 0.60 mL 35.45 mL 34. 85 mc Trial 2 20.00 mL 0.85 mL 34. 30 mL Trial 3 20.00 mL 0.45mL 33.80mL
Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.26QAP
Related questions
Question
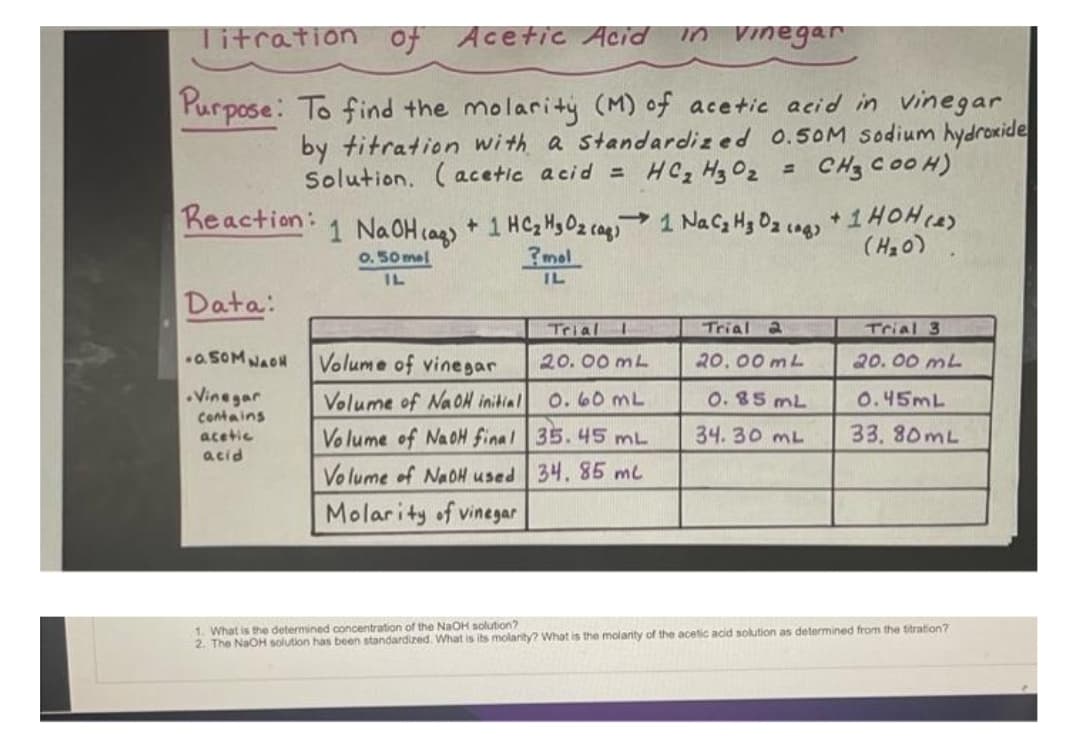
Transcribed Image Text:Titration of Acetic Acid
in vinegar
Purpose: To find the molarity (M) of acetic acid in vinegar
by titration with a standardized 0.50M sodium hydroxide
Solution. (acetic acid = HC₂ H3 0₂ = CH3 COOH)
Reaction:
Data:
-0.50M NAOH
.Vinegar
contains
acetic
acid
1 NaOH(aq) + 1 HC₂ H₂ Oz (ag)
0.50 mel
?mol
IL
IL
Volume of vinegar
Volume of NaOH initial
->
Volume of NaoH final
Volume of NaOH used
Molarity of vinegar
Trial I
20.00 mL
0.60 mL
35.45 mL
34, 85 m²
1 Na C₂ Hz O₂ (g) + 1 HOH (e)
(H₂O)
Trial 2
20.00 mL
0.85 mL
34.30 ML
Trial 3
20.00 mL
0.45mL
33.80mL
1. What is the determined concentration of the NaOH solution?
2. The NaOH solution has been standardized. What is its molarity? What is the molarity of the acetic acid solution as determined from the titration?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



