Treating 1,3-butadiene with 1 mole of HBr gives a mixture of two isomeric products. Br CH2=CH-CH=CH, + H-Br - CH,— CH—CH— CH, + CH,—Сн— СН—CH, — Вr 1,3-Butadiene 3-Bromo-1-butene 1-Bromo-2-butene (a racemic mixture) Propose a mechanism that accounts for the formation of these two products.
Treating 1,3-butadiene with 1 mole of HBr gives a mixture of two isomeric products. Br CH2=CH-CH=CH, + H-Br - CH,— CH—CH— CH, + CH,—Сн— СН—CH, — Вr 1,3-Butadiene 3-Bromo-1-butene 1-Bromo-2-butene (a racemic mixture) Propose a mechanism that accounts for the formation of these two products.
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 34MP: Reaction of iodoethane with CN- yields a small amount of isonitrile, CH3CH2N≡C, along with the...
Related questions
Question
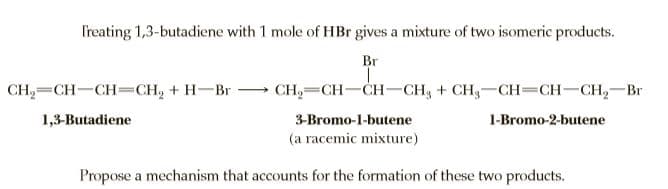
Transcribed Image Text:Treating 1,3-butadiene with 1 mole of HBr gives a mixture of two isomeric products.
Br
CH2=CH-CH=CH, + H-Br -
CH,— CH—CH— CH, + CH,—Сн— СН—CH, — Вr
1,3-Butadiene
3-Bromo-1-butene
1-Bromo-2-butene
(a racemic mixture)
Propose a mechanism that accounts for the formation of these two products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning