TRIA TRIAL 1 TRIAL 2 (if nece Mass of metal 0.0301 0.0294 Final burette reading 25.40 2a.50 Initial burette reading 0.04 0.42 Volume of H, 25.36 29.08 Mass of Metal Volume H, Ratio of % difference between Trial 1 and Trial 2 (show calculation) Additional % difference calculations (if necessary) Indicate which results (if any) were discarded and why Room temperature: 759.2 Atmospheric pressure: 48.4 26.8 Vapour pressure H,0:
TRIA TRIAL 1 TRIAL 2 (if nece Mass of metal 0.0301 0.0294 Final burette reading 25.40 2a.50 Initial burette reading 0.04 0.42 Volume of H, 25.36 29.08 Mass of Metal Volume H, Ratio of % difference between Trial 1 and Trial 2 (show calculation) Additional % difference calculations (if necessary) Indicate which results (if any) were discarded and why Room temperature: 759.2 Atmospheric pressure: 48.4 26.8 Vapour pressure H,0:
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
3
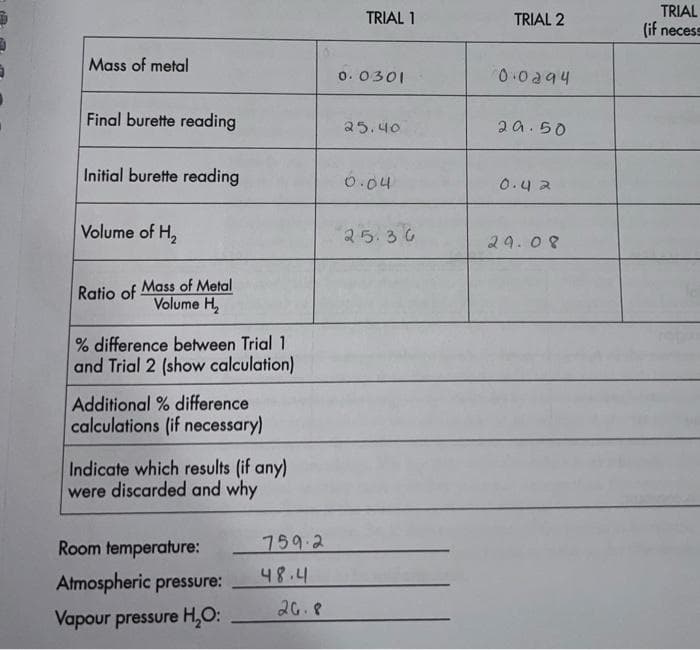
Transcribed Image Text:TRIAL
(if necess
TRIAL 1
TRIAL 2
Mass of metal
o. 0301
0.0a94
Final burette reading
25.40
2a.50
Initial burette reading
0.04
0.42
Volume of H,
25.36
29.08
Ratio of Mass of Metal
Volume H
% difference between Trial 1
and Trial 2 (show calculation)
Additional % difference
calculations (if necessary)
Indicate which results (if any)
were discarded and why
Room temperature:
759.2
48.4
Atmospheric pressure:
26.8
Vapour pressure H,O:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning