Trial 1 Trial 2 Trial 3 Moles HCI (mols) (Use the balanced equation to 7. create a mole ratio to convert moles of NaOH to moles of HCI) 0.00267168 0.00271584 0.00272688 Part 6 of 10 Trial 1 Trial 2 Trial 3 0.02420 0.0284 Volume of HCL (L) 8. |(1L = 1000 mL) 0.02500 * 0.02500 e 0.02500 a 0.02500 Part 7 of 10 Trial 1 Trial 2 Trial 3 Molarity HCl (M) moles solute 9. (molarity liters solution
Trial 1 Trial 2 Trial 3 Moles HCI (mols) (Use the balanced equation to 7. create a mole ratio to convert moles of NaOH to moles of HCI) 0.00267168 0.00271584 0.00272688 Part 6 of 10 Trial 1 Trial 2 Trial 3 0.02420 0.0284 Volume of HCL (L) 8. |(1L = 1000 mL) 0.02500 * 0.02500 e 0.02500 a 0.02500 Part 7 of 10 Trial 1 Trial 2 Trial 3 Molarity HCl (M) moles solute 9. (molarity liters solution
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 49QAP: Caffeine is made up of 49.5% C, 5.2% H, 16.5% O, and 28.9% N. A solution made up of 8.25 g of...
Related questions
Question
Find Molarity for HCl for Trials one through three
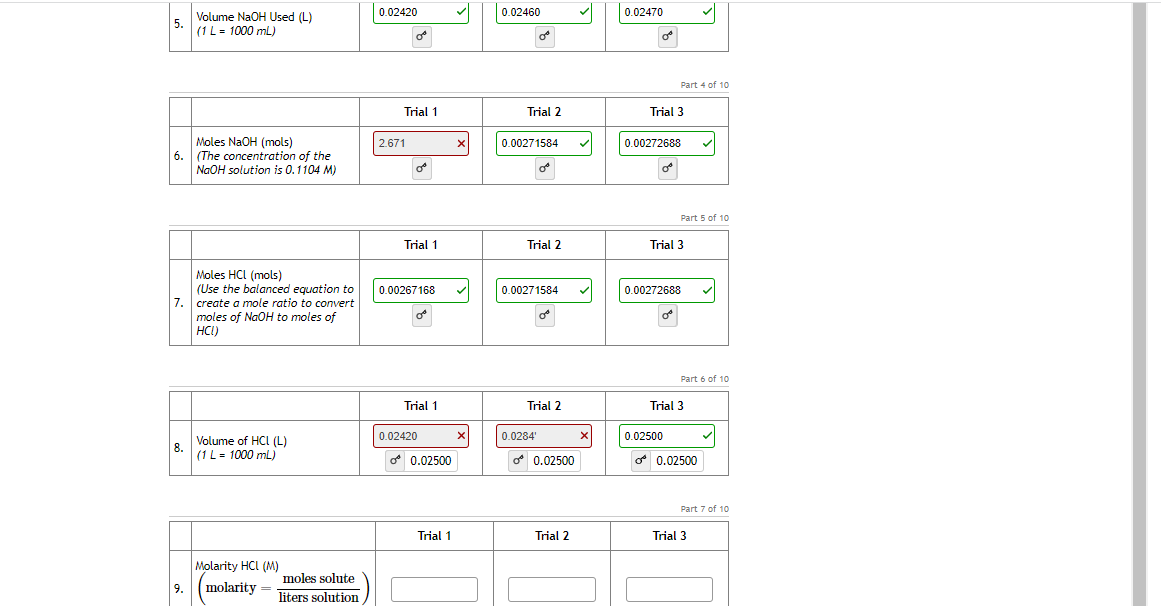
Transcribed Image Text:0.02420
0.02460
0.02470
Volume NaOH Used (L)
5.
(1L = 1000 mL)
Part 4 of 1o
Trial 1
Trial 2
Trial 3
Moles NaOH (mols)
2.671
0.00271584
0.00272688
6.
(The concentration of the
NaOH solution is 0.1104 M)
Part 5 of 10
Trial 1
Trial 2
Trial 3
Moles HCI (mols)
(Use the balanced equation to
7. create a mole ratio to convert
moles of NaOH to moles of
HCI)
0.00267168
0.00271584
0.00272688
Part 6 of 10
Trial 1
Trial 2
Trial 3
0.02420
0.0284'
x
0.02500
8.
Volume of HCL (L)
(1L = 1000 mL)
d 0.02500
o 0.02500
O 0.02500
Part 7 of 10
Trial 1
Trial 2
Trial 3
Molarity HCl (M)
moles solute
9.
molarity
liters solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
