Trinitrotoluene (TNT) is a highly explosive compound with a molecular weight of 227 It mol kJ -. If someone mol has an enthalpy of fusion of 40.8 were to melt 300 grams of TNT at its melting point of 80.3°C, how many government watchlists would they end change in entropy? on? What would be the associated up If 300 of liquid TNT were to solidify at 80.3°C, what would be the change in entropy? grams
Trinitrotoluene (TNT) is a highly explosive compound with a molecular weight of 227 It mol kJ -. If someone mol has an enthalpy of fusion of 40.8 were to melt 300 grams of TNT at its melting point of 80.3°C, how many government watchlists would they end change in entropy? on? What would be the associated up If 300 of liquid TNT were to solidify at 80.3°C, what would be the change in entropy? grams
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 93QRT: The standard molar entropy of iodine vapor, I2(g), is 260.7 J Kl mol-1 and the standard molar...
Related questions
Question
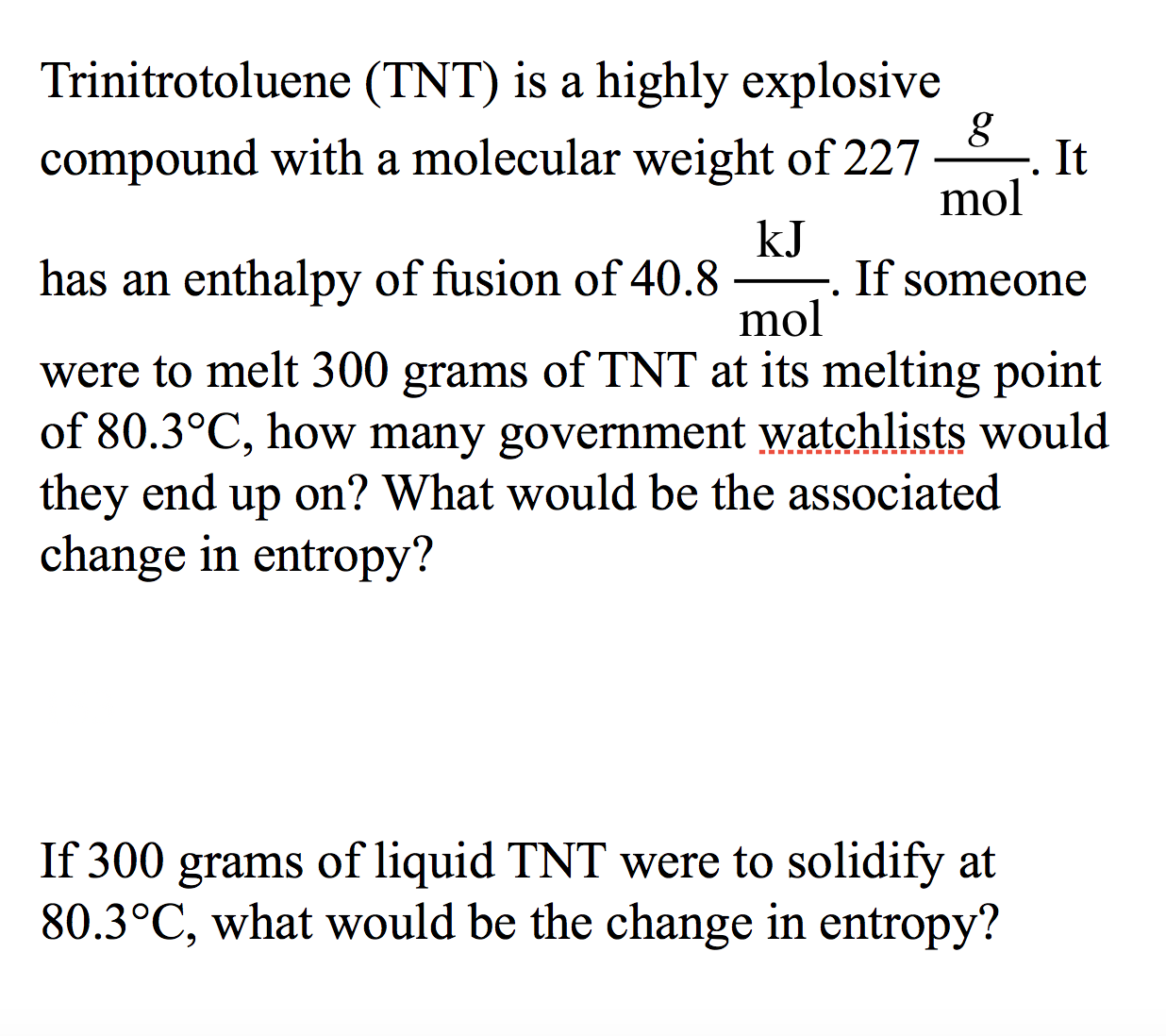
Transcribed Image Text:Trinitrotoluene (TNT) is a highly explosive
compound with a molecular weight of 227
It
mol
kJ
-. If someone
mol
has an enthalpy of fusion of 40.8
were to melt 300 grams of TNT at its melting point
of 80.3°C, how many government watchlists would
they end
change in entropy?
on? What would be the associated
up
If 300
of liquid TNT were to solidify at
80.3°C, what would be the change in entropy?
grams
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning