true or false?
When the reaction quotient is greater than the equilibrium constant (Qc>Kc) the reaction will proceed in reverse
A typical reversible reaction is given below:
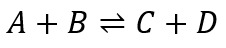
At equilibrium, the equilibrium constant of this reaction is given by:
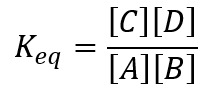
At equilibrium, the concentration fo all the species are constant because the rate of forward and reverse reactions are equal.
If the reaction is not at equilibrium than the ratio of the concentration of product to reactants if given by reaction quotient.
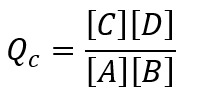
If the value of the reaction quotient is higher than the equilibrium constant (Qc>Kc) than we can say that the concentration of the products are higher than the equilibrium condition and the concentration of the reactants are lower than the equilibrium condition.

If concentration of products are higher than the equilibrium condition the rate of thereverse reaction is higher than the rate of forward reaction. Thus the reaction will process in reverse.
Step by step
Solved in 3 steps with 4 images









