TRUE or FALSE. Write TRUE if the statement is correct and FALSE if the statement is wrong. 1. Batteries can be recharged use an outside power Source to reverse electrode reactions. Those that cannot be recharged use redox reactions that cannot be easily reversed. 2. The automobile battery is the best example of a lead storage battery. 3. For lead storage battery, the anode and cathode are both immersed in the electrolyte sulfuric acid, which is commonly called battery acid. 4. The most common fuel cell is the hydrogen – oxygen fuel cell, where H2 is pumped into the anode and O2 is directed into the cathode. 5. Primary batteries are single-use batteries because they can be recharged.
TRUE or FALSE. Write TRUE if the statement is correct and FALSE if the statement is wrong. 1. Batteries can be recharged use an outside power Source to reverse electrode reactions. Those that cannot be recharged use redox reactions that cannot be easily reversed. 2. The automobile battery is the best example of a lead storage battery. 3. For lead storage battery, the anode and cathode are both immersed in the electrolyte sulfuric acid, which is commonly called battery acid. 4. The most common fuel cell is the hydrogen – oxygen fuel cell, where H2 is pumped into the anode and O2 is directed into the cathode. 5. Primary batteries are single-use batteries because they can be recharged.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 122AE
Related questions
Question
please answer 1-5. I really really need answer for this but this is my last question for this month so thank you if you can help me :(
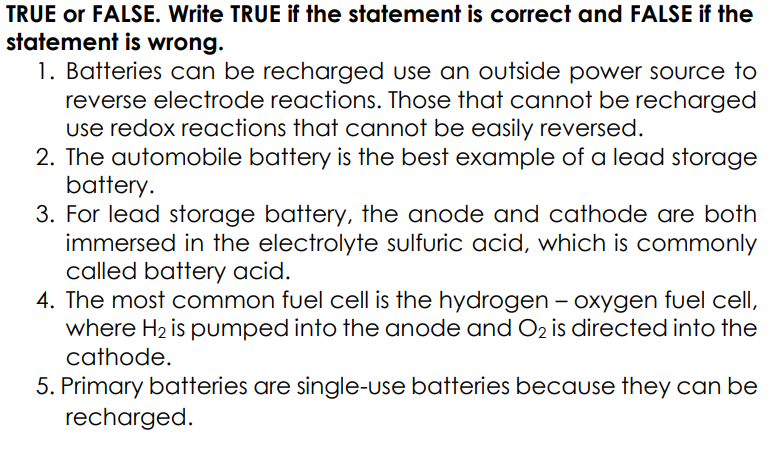
Transcribed Image Text:TRUE or FALSE. Write TRUE if the statement is correct and FALSE if the
statement is wrong.
1. Batteries can be recharged use an outside power Source to
reverse electrode reactions. Those that cannot be recharged
use redox reactions that cannot be easily reversed.
2. The automobile battery is the best example of a lead storage
battery.
3. For lead storage battery, the anode and cathode are both
immersed in the electrolyte sulfuric acid, which is commonly
called battery acid.
4. The most common fuel cell is the hydrogen – oxygen fuel cell,
where H2 is pumped into the anode and O2 is directed into the
cathode.
5. Primary batteries are single-use batteries because they can be
recharged.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning