What to do: Read each statement below and identify if it defines electrode potential, reduction potential, oxidation potential or cell potential. Write your answers on a separate sheet of раper. 1. It refers to the difference of equilibrium potential between the surrounding solution and the metal electrode. 2. It is the reaction that takes place at the cathode. 3. It is the reaction that takes place at the anode. 4. It is the ability of anion solution to become reduced picking up or gaining electrons from its own electrode. 5. It is the ability of an electrode to become oxidized giving up or losing electrons. 6. It refers to the potential difference between the electrodes corresponding to an external electron flow from anode to cathode. 7. It is the combination of reduction potential at the cathode and oxidation potential at the anode. 8. An example of this reaction is Zns) 9. An example of this reaction is Cu*?jaq) + 2e- 10. It is illustrated as: Ecell = Eox,Anode + Ered,Cathode Znaq) + 2e- . » Cus) -
What to do: Read each statement below and identify if it defines electrode potential, reduction potential, oxidation potential or cell potential. Write your answers on a separate sheet of раper. 1. It refers to the difference of equilibrium potential between the surrounding solution and the metal electrode. 2. It is the reaction that takes place at the cathode. 3. It is the reaction that takes place at the anode. 4. It is the ability of anion solution to become reduced picking up or gaining electrons from its own electrode. 5. It is the ability of an electrode to become oxidized giving up or losing electrons. 6. It refers to the potential difference between the electrodes corresponding to an external electron flow from anode to cathode. 7. It is the combination of reduction potential at the cathode and oxidation potential at the anode. 8. An example of this reaction is Zns) 9. An example of this reaction is Cu*?jaq) + 2e- 10. It is illustrated as: Ecell = Eox,Anode + Ered,Cathode Znaq) + 2e- . » Cus) -
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 22E: The mass of three different metal electrodes, each from a different galvanic cell, were determined...
Related questions
Question
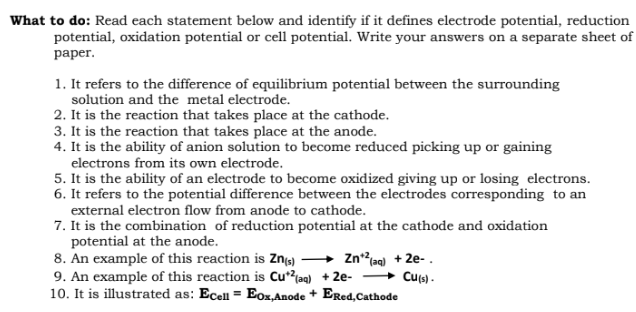
Transcribed Image Text:What to do: Read each statement below and identify if it defines electrode potential, reduction
potential, oxidation potential or cell potential. Write your answers on a separate sheet of
раper.
1. It refers to the difference of equilibrium potential between the surrounding
solution and the metal electrode.
2. It is the reaction that takes place at the cathode.
3. It is the reaction that takes place at the anode.
4. It is the ability of anion solution to become reduced picking up or gaining
electrons from its own electrode.
5. It is the ability of an electrode to become oxidized giving up or losing electrons.
6. It refers to the potential difference between the electrodes corresponding to an
external electron flow from anode to cathode.
7. It is the combination of reduction potential at the cathode and oxidation
potential at the anode.
8. An example of this reaction is Zns)
9. An example of this reaction is Cu*?laq) + 2e-
10. It is illustrated as: Ecell = Eox,Anode + Ered,Cathode
Znag) + 2e-.
» Cus) .
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning