Tungsten trioxide (WO,) has a rich yellow color and is often used as a pigment in ceramics and paints. To test a ceramic vase for its WO, content, a 10.15 g sample of the vase was ground and reduced with Pb(Hg) to convert any WO, to W3+. The resulting W3+ was transferred to 500.0 mL of 1.00 M HCI. A 100.00 mL aliquot of the HCl solution required 14.78 mL of 0.08271 M potassium permanganate (KMNO,) to reach the purple endpoint. A blank required 0.26 mL. Balance the redox reaction. redox reaction: |MnO + w3+ Mn2+ + WO3+ Determine the percent WO, in the ceramic sample. percent WO3: 70
Tungsten trioxide (WO,) has a rich yellow color and is often used as a pigment in ceramics and paints. To test a ceramic vase for its WO, content, a 10.15 g sample of the vase was ground and reduced with Pb(Hg) to convert any WO, to W3+. The resulting W3+ was transferred to 500.0 mL of 1.00 M HCI. A 100.00 mL aliquot of the HCl solution required 14.78 mL of 0.08271 M potassium permanganate (KMNO,) to reach the purple endpoint. A blank required 0.26 mL. Balance the redox reaction. redox reaction: |MnO + w3+ Mn2+ + WO3+ Determine the percent WO, in the ceramic sample. percent WO3: 70
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 158IP: The vanadium in a sample of ore is converted to VO2+. The VO2+ ion is subsequently titrated with...
Related questions
Question
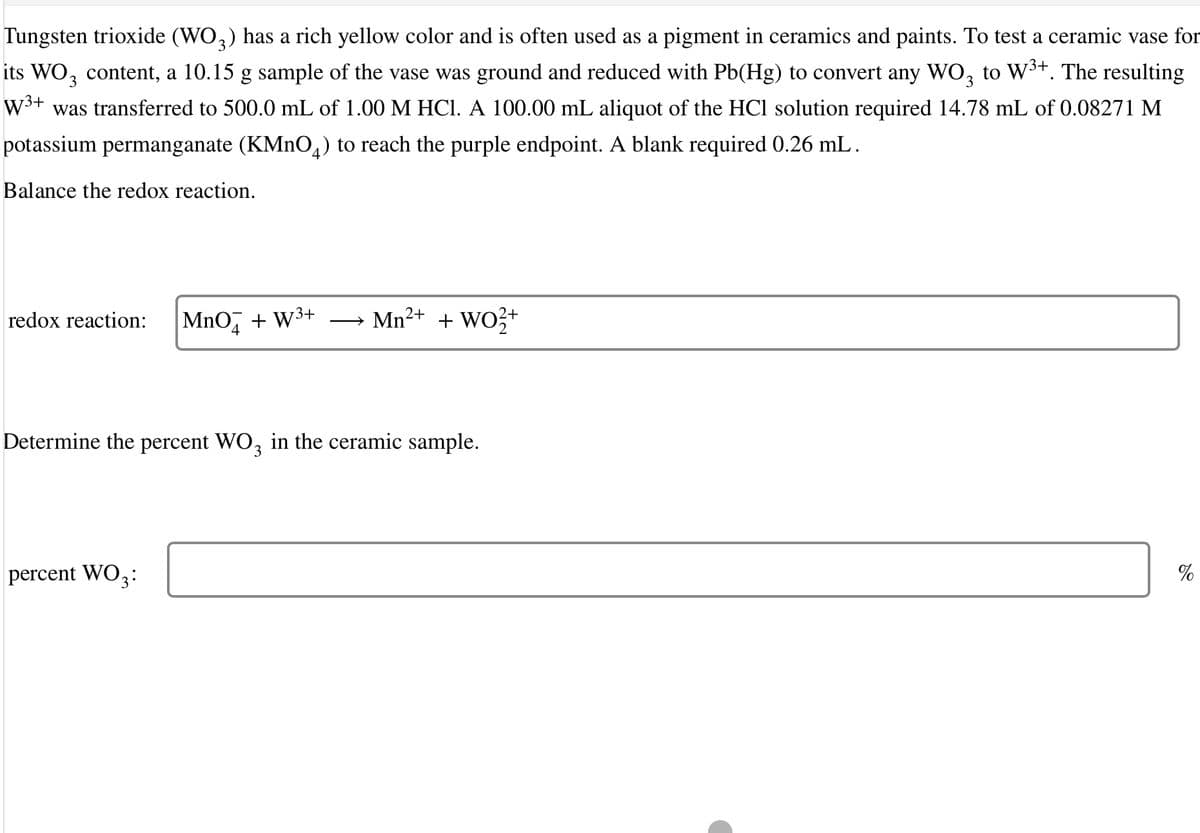
Transcribed Image Text:Tungsten trioxide (WO,) has a rich yellow color and is often used as a pigment in ceramics and paints. To test a ceramic vase for
its WO, content, a 10.15 g sample of the vase was ground and reduced with Pb(Hg) to convert any WO, to W³+. The resulting
W3+
was transferred to 500.0 mL of 1.00 M HCI. A 100.00 mL aliquot of the HCl solution required 14.78 mL of 0.08271 M
potassium permanganate (KMNO,) to reach the purple endpoint. A blank required 0.26 mL.
Balance the redox reaction.
redox reaction:
MnO,
+ W3+
Mn2+ + WO3+
Determine the percent WO, in the ceramic sample.
percent WO3:
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning