Two moles of ideal helium gas are in a rubber balloon at 30 0 C. The balloon is fully expandable and can be assumed to require no energy in its expansion. The temperature of the gas in the balloon is slowly changed to 35 0 C. The amount of heat required in raising the temperature is nearly (take R = 8.3 J/mol .K)
Two moles of ideal helium gas are in a rubber balloon at 30 0 C. The balloon is fully expandable and can be assumed to require no energy in its expansion. The temperature of the gas in the balloon is slowly changed to 35 0 C. The amount of heat required in raising the temperature is nearly (take R = 8.3 J/mol .K)
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter17: Energy In Thermal Processes: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 31P: An ideal gas initially at 300 K undergoes an isobaric expansion at 2.50 kPa. If the volume increases...
Related questions
Question
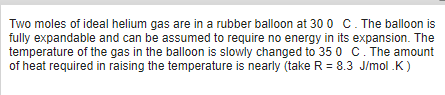
Transcribed Image Text:Two moles of ideal helium gas are in a rubber balloon at 30 0 C. The balloon is
fully expandable and can be assumed to require no energy in its expansion. The
temperature of the gas in the balloon is slowly changed to 35 0 C. The amount
of heat required in raising the temperature is nearly (take R = 8.3 J/mol .K)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning