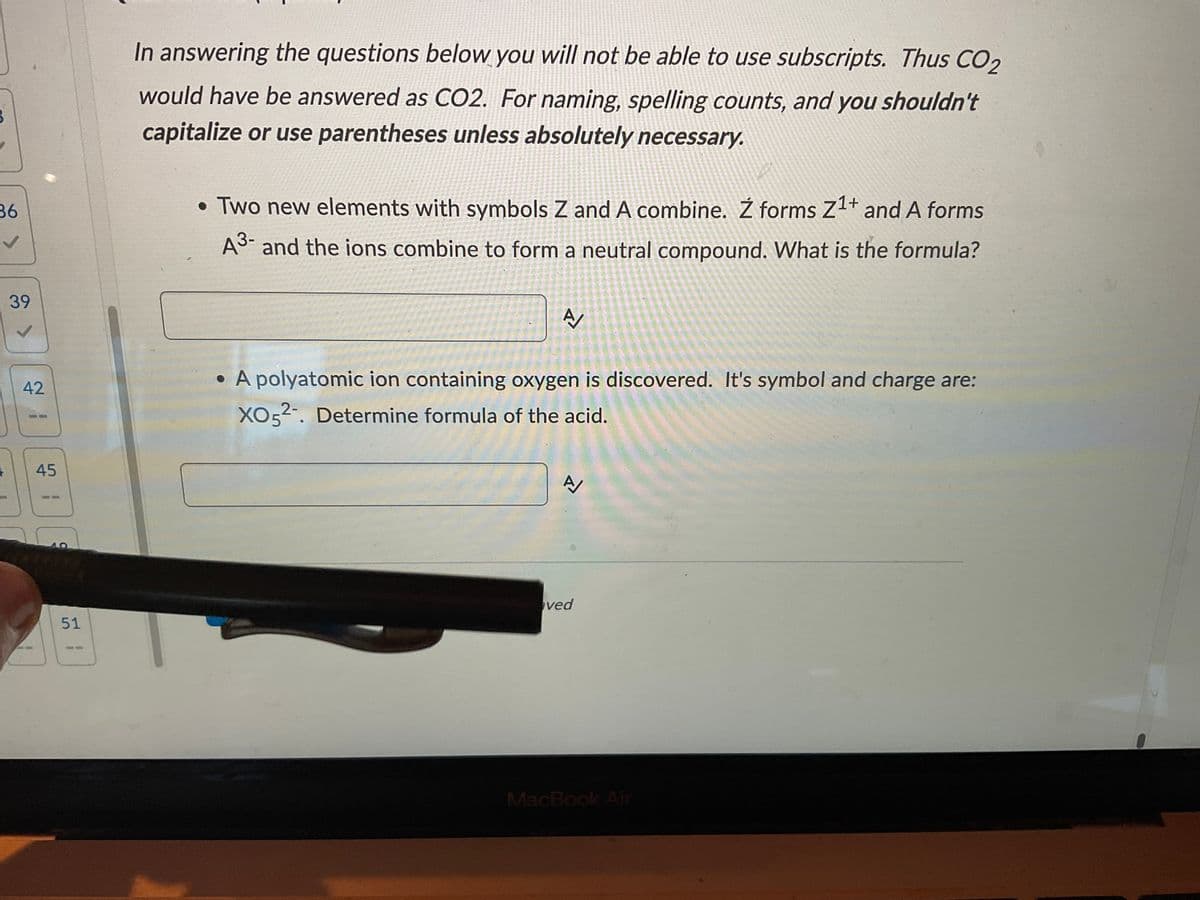
• Two new elements with symbols Z and A combine. Ź forms Z¹+ and A forms A3- and the ions combine to form a neutral compound. What is the formula? A/ • A polyatomic ion containing oxygen is discovered. It's symbol and charge are: XO52-. Determine formula of the acid.
• Two new elements with symbols Z and A combine. Ź forms Z¹+ and A forms A3- and the ions combine to form a neutral compound. What is the formula? A/ • A polyatomic ion containing oxygen is discovered. It's symbol and charge are: XO52-. Determine formula of the acid.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.6PAE: One application of conductive polymers is in photovoltaic solar cells. Such devices have...
Related questions
Question
Transcribed Image Text:36
V
+
39
42
45
40
51
In answering the questions below you will not be able to use subscripts. Thus CO₂
would have be answered as CO2. For naming, spelling counts, and you shouldn't
capitalize or use parentheses unless absolutely necessary.
• Two new elements with symbols Z and A combine. Ź forms Z¹+ and A forms
A³- and the ions combine to form a neutral compound. What is the formula?
A/
• A polyatomic ion containing oxygen is discovered. It's symbol and charge are:
XO52. Determine formula of the acid.
A/
ved
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning