Two sets of lonizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required.
Two sets of lonizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 39P: Without consulting any tables, arrange the following substances in order and explain your choice of...
Related questions
Question
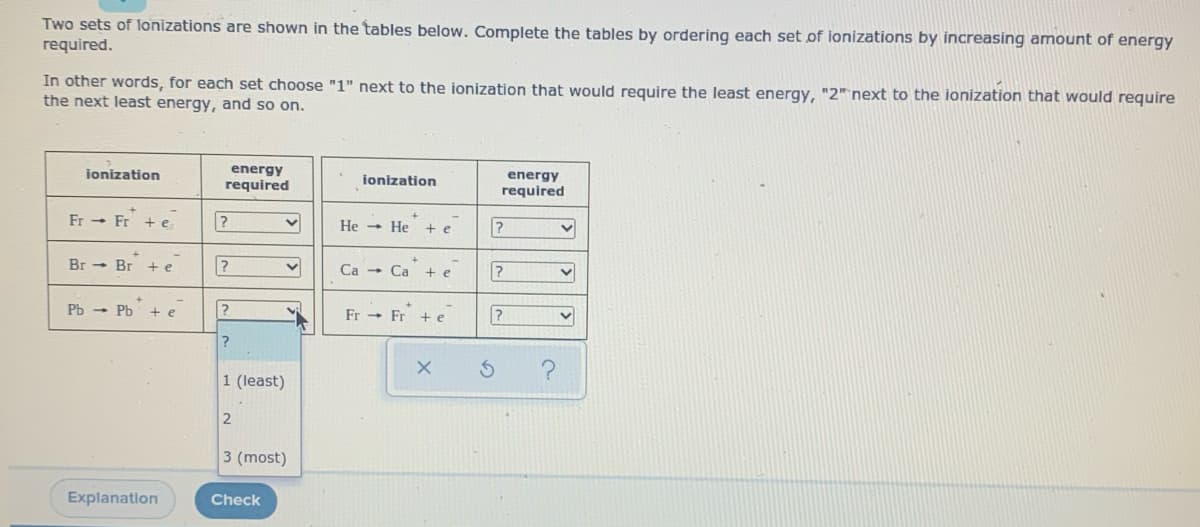
Transcribed Image Text:Two sets of lonizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy
required.
In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require
the next least energy, and so on.
energy
required
ionization
energy
required
ionization
Fr - Fr + e
He - He +e
Br - Br + e
Ca - Ca
+ e
Pb - Pb + e
Fr - Fr + e
1?
1 (least)
2
3 (most)
Explanation
Check
Expert Solution
Step 1
Ionisation energy is the amount of energy required to take out an electron from the outermost shell of an element in gaseous state.
In the first set Bromine has the most value as it will rather accept one electron to gain fulfilled electronic configuration than remove one electron. Electronic configuration: [Ar]4s23d104p5
And Francium has the least value as it will easily remove one electron to acquire noble gas configuration which is very stable. Electronic configuration: [Rn]7s1
So in the first set,
For Fr : 1(least)
For Br: 3(most)
For Pb: 2
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning