Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on.
Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter8: Electron Configurations And Periodicity
Section: Chapter Questions
Problem 8.29QP: Periodic Properties I A hypothetical element, X, has the following ionization energy values: First...
Related questions
Question
100%
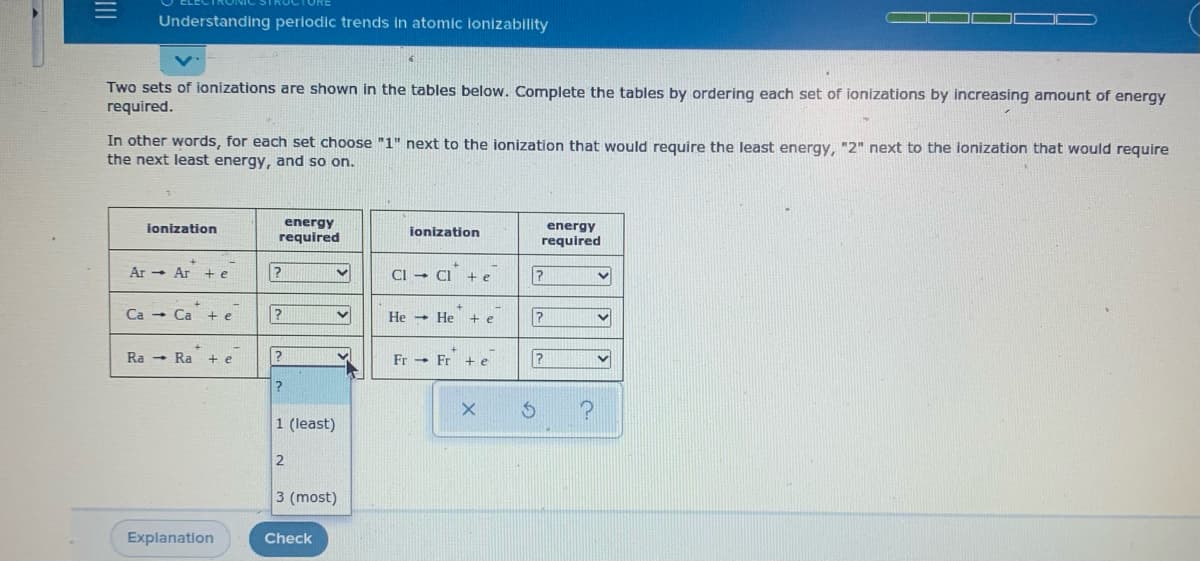
Transcribed Image Text:Understanding periodic trends in atomic lonizability
Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy
required.
In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require
the next least energy, and so on.
energy
required
ionization
jonization
energy
required
Ar - Ar + e
CI - CI
Ca - Ca
+ e
He He
+ e
Ra - Ra + e
Fr - Fr + e
1 (least)
2
3 (most)
Explanation
Check
II
Expert Solution
Step 1 Ionization energy
Since we know that Ionization energy is the amount of energy needed to remove an electron from an atom.
- Ionization energy decreases as we go down a group.
- Ionization energy increases from left to right across the periodic table.i.e period
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning