Two sets of lonizations are shown in the tables below. Complete thne tables by ordering each set of lonizations by Increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. energy required energy required ionization ionization Cs - Cs + e Se + Se + e He - He + e CI + CI +e ? Rb - Rb + e v? Te - Te + e 1 (least) 2 ? 3 (most)
Two sets of lonizations are shown in the tables below. Complete thne tables by ordering each set of lonizations by Increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. energy required energy required ionization ionization Cs - Cs + e Se + Se + e He - He + e CI + CI +e ? Rb - Rb + e v? Te - Te + e 1 (least) 2 ? 3 (most)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 117QRT
Related questions
Question
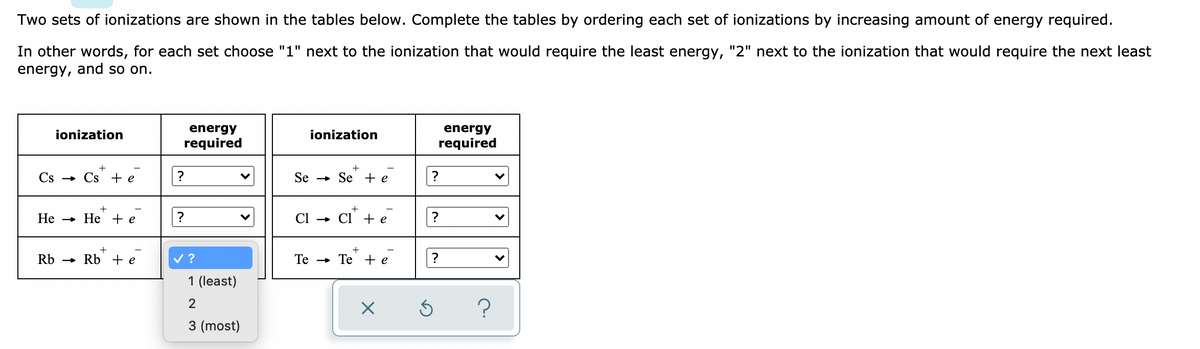
Transcribed Image Text:Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required.
In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least
energy, and so on.
ionization
energy
ionization
energy
required
required
Cs - Cs + e
Se
» Se - e
Не — Не +e
?
Ci
Cl' + e
Rb - Rb + e
v ?
Те — Те +e
?
1 (least)
3 (most)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning