Order of electron 1st 2nd Зrd 4th 5th 6th 7th 8th removed Ionisation energy for X/kJ mol- Ionisation energy for Y/kJ mol- 1000 2252 3363 4556 7004 8496 27108 31724 1314 3388 5301 7469 10990| 13327 71330 | 84078 The proton number of elements X and Y is less than 20. Which of the following statements is true? A. X forms an ionic chloride. B. The atomic radius of X is smaller than Y. C. X combines with Y to form a linear molecule. D. X and Y belong to the same group in the Periodic Table.
Order of electron 1st 2nd Зrd 4th 5th 6th 7th 8th removed Ionisation energy for X/kJ mol- Ionisation energy for Y/kJ mol- 1000 2252 3363 4556 7004 8496 27108 31724 1314 3388 5301 7469 10990| 13327 71330 | 84078 The proton number of elements X and Y is less than 20. Which of the following statements is true? A. X forms an ionic chloride. B. The atomic radius of X is smaller than Y. C. X combines with Y to form a linear molecule. D. X and Y belong to the same group in the Periodic Table.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 87AP: At large interatomic separations, an alkali halide molecule MX has a lower energy as two neutral...
Related questions
Question
q17
Thanks for your help ! :D
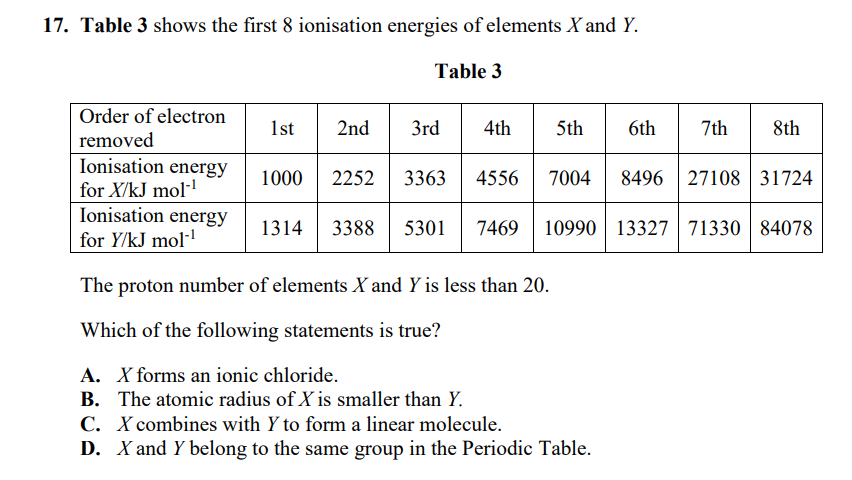
Transcribed Image Text:17. Table 3 shows the first 8 ionisation energies of elements X and Y.
Table 3
Order of electron
1st
2nd
3rd
4th
5th
6th
7th
8th
removed
Ionisation energy
for X/kJ mol-
Ionisation energy
for Y/kJ mol-
1000
2252
3363
4556
7004
8496 27108 31724
1314
3388
5301
7469
10990 13327 71330 84078
The proton number of elements X and Y is less than 20.
Which of the following statements is true?
A. X forms an ionic chloride.
B. The atomic radius of X is smaller than Y.
C. X combines with Y to form a linear molecule.
D. X and Y belong to the same group in the Periodic Table.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning