Two trials were completed in a kinetics experiment, resulting in the data collected below. Calculate the rate of each trial using the change iodine concentration: rate = Enter your answers in the boxes, rounded appropriately for significant figures. Use scientific notation, with E in the place of x10. For example, 1234 would be entered as 1.234E3 and 0.00123 would be entered as 1.23E-3. Do not use units. Trial Acetone, M 2. M H*1, M Time (sec) Rate 1 1.8 0.0025 0.33 136 2 1.8 0.0025 0.66 71 What are the units of rate? Use symbols only your answer. What is the order of the reaction with respect to hydrogen ion? Report your answer to the tenths place. What is the order of the reaction with respect to hydrogen ion rounded to the nearest whole number?
Two trials were completed in a kinetics experiment, resulting in the data collected below. Calculate the rate of each trial using the change iodine concentration: rate = Enter your answers in the boxes, rounded appropriately for significant figures. Use scientific notation, with E in the place of x10. For example, 1234 would be entered as 1.234E3 and 0.00123 would be entered as 1.23E-3. Do not use units. Trial Acetone, M 2. M H*1, M Time (sec) Rate 1 1.8 0.0025 0.33 136 2 1.8 0.0025 0.66 71 What are the units of rate? Use symbols only your answer. What is the order of the reaction with respect to hydrogen ion? Report your answer to the tenths place. What is the order of the reaction with respect to hydrogen ion rounded to the nearest whole number?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 4QAP: Consider the following hypothetical reaction: 2AB2(g)A2(g)+2B2(g)A 500.0-mL flask is filled with...
Related questions
Question
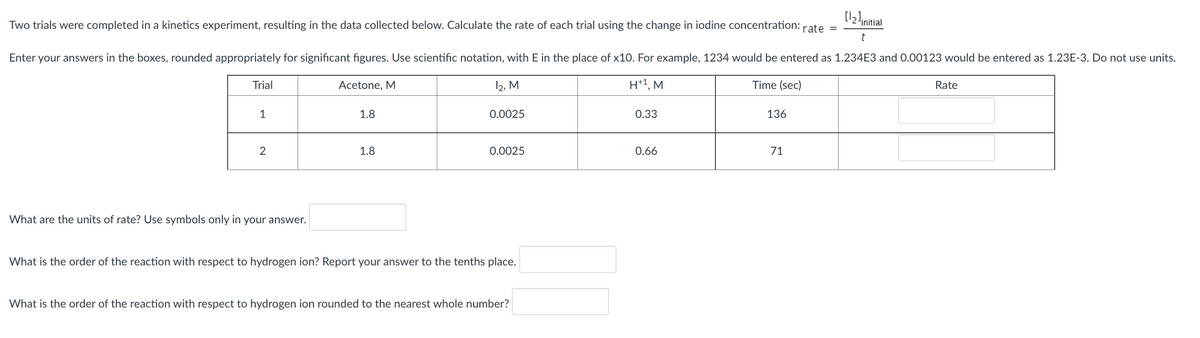
Transcribed Image Text:[12!initial
Two trials were completed in a kinetics experiment, resulting in the data collected below. Calculate the rate of each trial using the change in iodine concentration:
rate
Enter your answers in the boxes, rounded appropriately for significant figures. Use scientific notation, with E in the place of x10. For example, 1234 would be entered as 1.234E3 and 0.00123 would be entered as 1.23E-3. Do not use units.
Trial
Acetone, M
12, M
H*1, M
Time (sec)
Rate
1
1.8
0.0025
0.33
136
2
1.8
0.0025
0.66
71
What are the units of rate? Use symbols only in your answer.
What is the order of the reaction with respect to hydrogen ion? Report your answer to the tenths place.
What is the order of the reaction with respect to hydrogen ion rounded to the nearest whole number?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning