uestion 28 The half-reaction occurring at the anode in the balanced reaction shown below is 3MNO 4 (aq) + 24H (aq) + 5Fe (5) → 3Mn 2+ (aq) + 5Fe 3+ (aq) + 12H 20 () O Fe (5) → Fe2+ (aq) + 2e O 2MN04 (aq) + 12H (aq) + 6e 2MN2+ (aq) + 3H20 (I) Fe2+ (aq) Fe3+ (aq) + e Fe (s) Fe3+ (aq) + 3e" O Mn04 (aq) + 8H* (aq) + 5e Mn* (aq) + 4H2O (I)
uestion 28 The half-reaction occurring at the anode in the balanced reaction shown below is 3MNO 4 (aq) + 24H (aq) + 5Fe (5) → 3Mn 2+ (aq) + 5Fe 3+ (aq) + 12H 20 () O Fe (5) → Fe2+ (aq) + 2e O 2MN04 (aq) + 12H (aq) + 6e 2MN2+ (aq) + 3H20 (I) Fe2+ (aq) Fe3+ (aq) + e Fe (s) Fe3+ (aq) + 3e" O Mn04 (aq) + 8H* (aq) + 5e Mn* (aq) + 4H2O (I)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.101QE: At 298 K, the solubility product constant for PbC2O4 is 8.5 1010, and the standard reduction...
Related questions
Question
100%
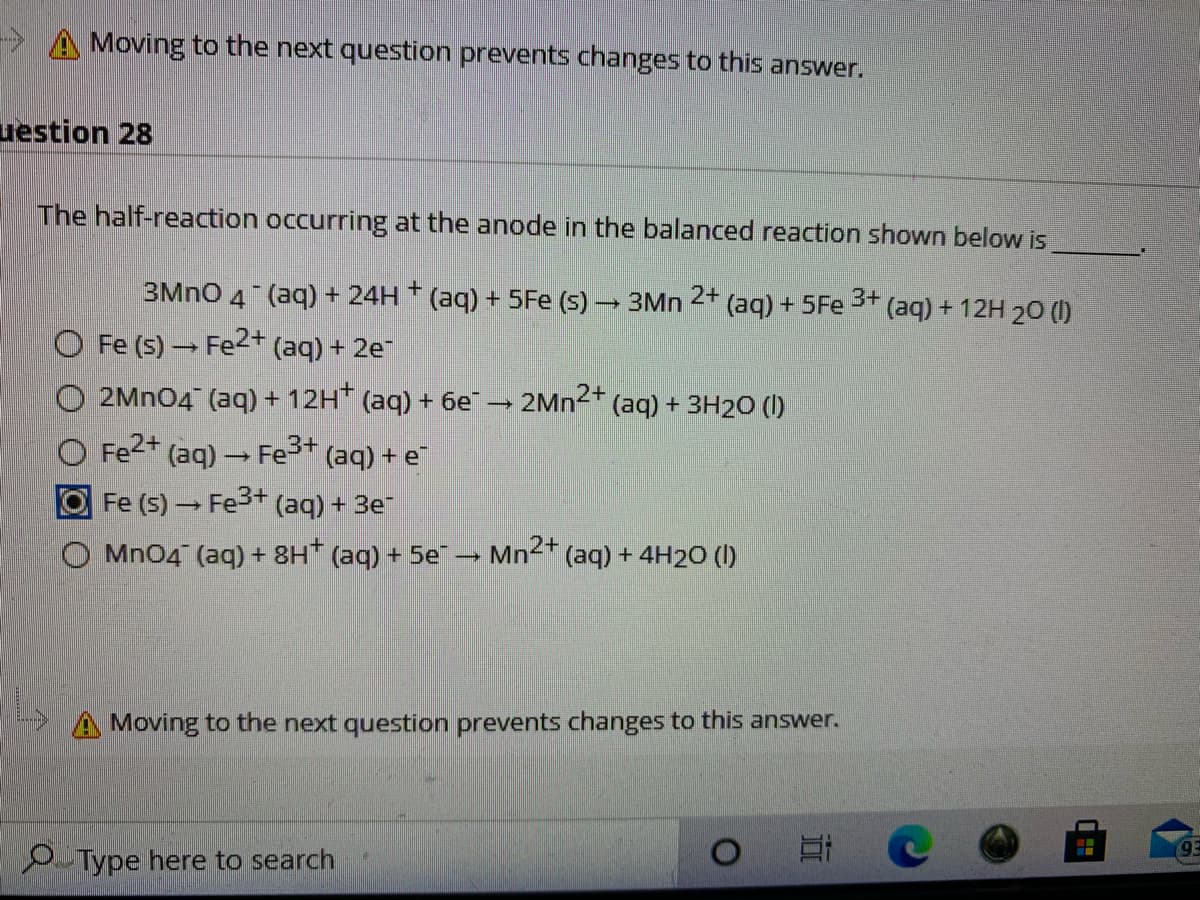
Transcribed Image Text:> A Moving to the next question prevents changes to this answer.
uestion 28
The half-reaction occurring at the anode in the balanced reaction shown below is
3MNO 4 (aq) + 24H * (aq) + 5Fe (s) → 3Mn
2+
3+
(aq) + 5Fe
(aq) + 12H 20 (O
O Fe (s) → Fe2+ (aq) + 2e
O 2MN04 (aq) + 12H (aq) + 6e
2MN2+
(aq) + 3H20 (1)
Fe2+ (aq) – Fe3+ (aq) + e
Fe (5) → Fe3+ (aq) + 3e"
O Mn04 (aq) + 8H* (aq) + 5e → Mn2+
(aq) + 4H20 (1)
A Moving to the next question prevents changes to this answer.
93
OType here to search
Expert Solution
Step 1
We know that in the half cell reaction
The reduction occurs at cathode and oxidation occurs at anode.
Based on this we can say that which is going to oxides in the half cell reaction in the above chemical equation.we can see that iron is oxidizing to Iron(III)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax