Use the following information to answer question 19. Students observed the Hoffman apparatus shown below while a concentrated solution of sodium chloride, NaCl(aq), was electrolyzed. The students observed bubbling around each electrode and also observed that a smaller volume of gas is collected in the tube above the anode than in the tube above the cathode. Electrolysis of NaCl(aq) Row 6.0 V A. B. C. D. Power supply Gas- Solution- Pt(s)- Oxygen Oxygen Hydrogen Hydrogen Cathode Gas Produced at the Cathode 19. Which of the following rows identifies the gases produced at each electrode? Gas -Solution Chlorine Hydrogen Chlorine Oxygen Gas Produced at the Anode Pt(s) Anode
Use the following information to answer question 19. Students observed the Hoffman apparatus shown below while a concentrated solution of sodium chloride, NaCl(aq), was electrolyzed. The students observed bubbling around each electrode and also observed that a smaller volume of gas is collected in the tube above the anode than in the tube above the cathode. Electrolysis of NaCl(aq) Row 6.0 V A. B. C. D. Power supply Gas- Solution- Pt(s)- Oxygen Oxygen Hydrogen Hydrogen Cathode Gas Produced at the Cathode 19. Which of the following rows identifies the gases produced at each electrode? Gas -Solution Chlorine Hydrogen Chlorine Oxygen Gas Produced at the Anode Pt(s) Anode
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.32QP: You have 1.0 M solutions of Al(NO3)3 and AgNO3 along with Al and Ag electrodes to construct a...
Related questions
Question
Need both of these
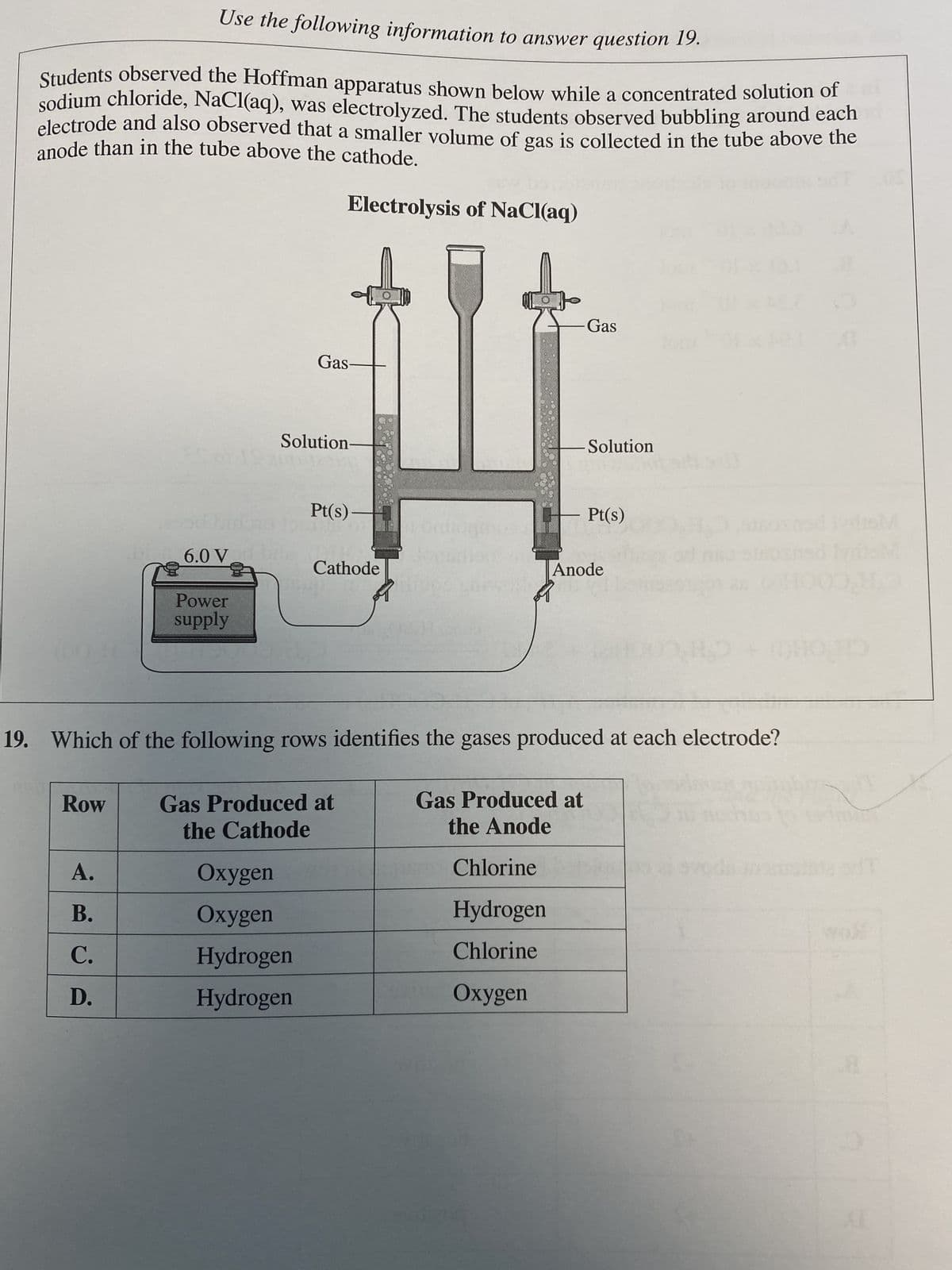
Transcribed Image Text:Use the following information to answer question 19.
Students observed the Hoffman apparatus shown below while a concentrated solution of
sodium chloride, NaCl(aq), was electrolyzed. The students observed bubbling around each
anode than in the tube above the cathode.
electrode and also observed that a smaller volume of gas is collected in the tube above the
(00:4
Row
6.0 V
A.
B.
C.
D.
Power
supply
Electrolysis of NaCl(aq)
Gas-
Solution-
Oxygen
Oxygen
Hydrogen
Hydrogen
Pt(s)
Cathode
Gas Produced at
the Cathode
19. Which of the following rows identifies the gases produced at each electrode?
Gas
Gas Produced at
the Anode
Chlorine
Hydrogen
Chlorine
Oxygen
Solution
90
Pt(s)
Anode
H
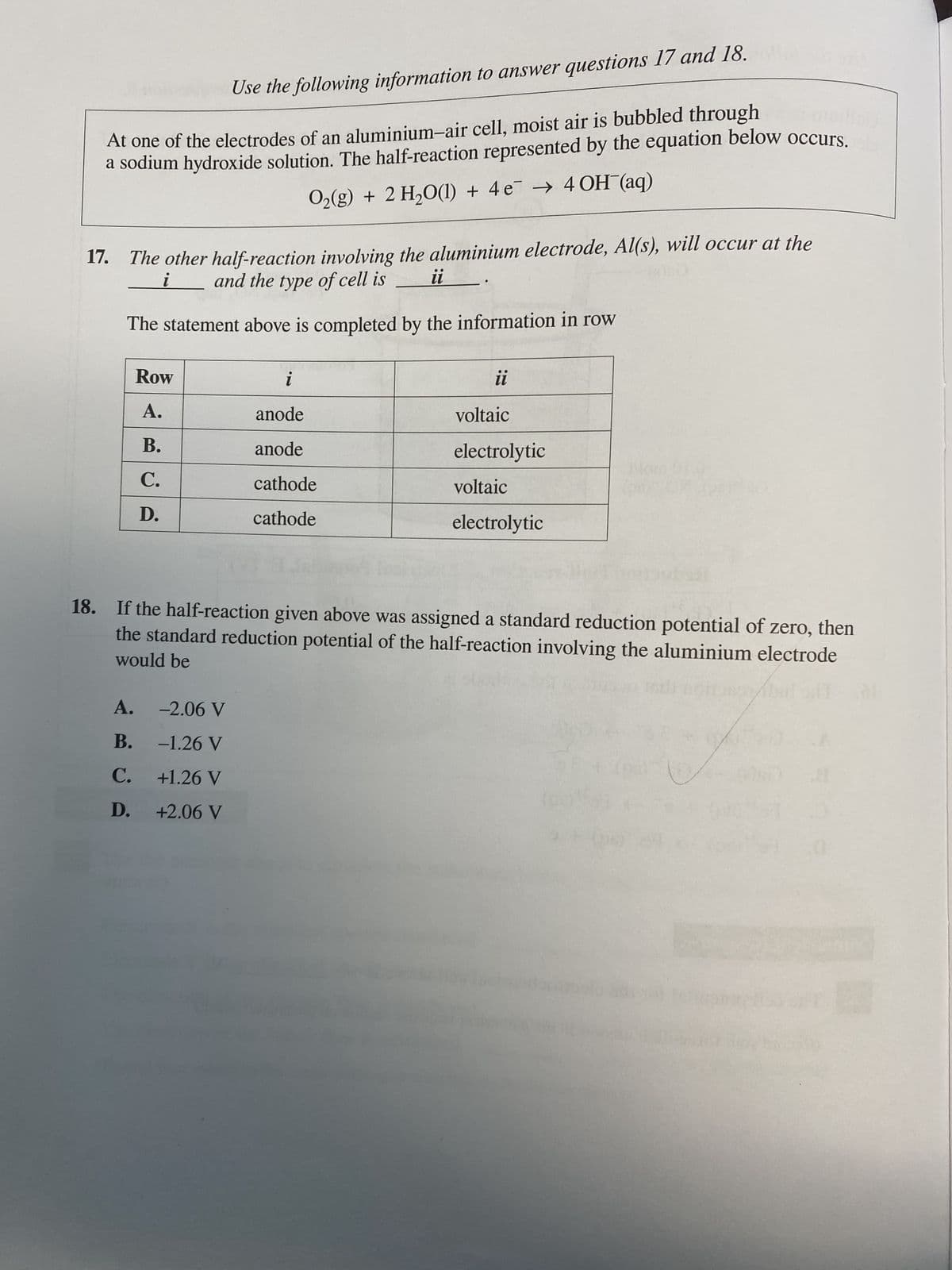
Transcribed Image Text:Use the following information to answer questions 17 and 18.
At one of the electrodes of an aluminium-air cell, moist air is bubbled through
a sodium hydroxide solution. The half-reaction represented by the equation below occurs.
Oz(g) + 2H,O(l) + 4e → 4OH (aq)
i
17. The other half-reaction involving the aluminium electrode, Al(s), will occur at the
and the type of cell is
ii
(3)0
The statement above is completed by the information in row
Row
A.
B.
C.
D.
i
anode
anode
cathode
cathode
A. -2.06 V
B.
-1.26 V
C.
+1.26 V
D.
+2.06 V
ii
voltaic
electrolytic
voltaic
electrolytic
18. If the half-reaction given above was assigned a standard reduction potential of zero, then
would be
the standard reduction potential of the half-reaction involving the aluminium electrode
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning