~60°C trial ~50°C trial ~40°C trial ~30°C trial ~20°C trial Vol. of HCl used (mL) 19.87 12.93 12.37 9.03 6.30 Moles of HCI used (mol) 9.94 6.47 6.19 4.52 3.15 Moles of borate present (mol) [Borate] (M) Ksp In(Ksp) 1/T (K-1)
~60°C trial ~50°C trial ~40°C trial ~30°C trial ~20°C trial Vol. of HCl used (mL) 19.87 12.93 12.37 9.03 6.30 Moles of HCI used (mol) 9.94 6.47 6.19 4.52 3.15 Moles of borate present (mol) [Borate] (M) Ksp In(Ksp) 1/T (K-1)
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 12P
Related questions
Question
Using the data provided in Table 1 to fill in the blanks on table 2
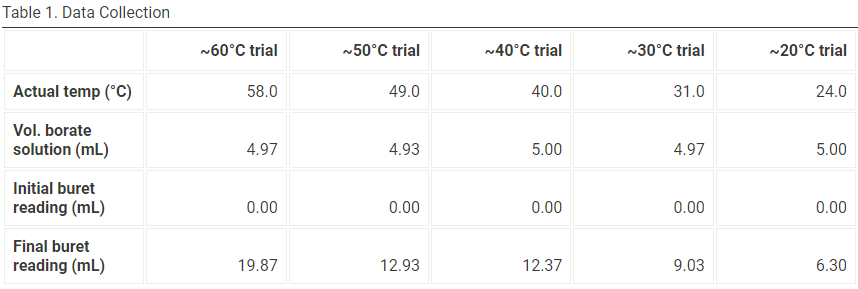
Transcribed Image Text:Table 1. Data Collection
~60°C trial
~50°C trial
~40°C trial
~30°C trial
~20°C trial
Actual temp (°C)
58.0
49.0
40.0
31.0
24.0
Vol. borate
solution (mL)
4.97
4.93
5.00
4.97
5.00
Initial buret
reading (mL)
0.00
0.00
0.00
0.00
0.00
Final buret
reading (mL)
19.87
12.93
12.37
9.03
6.30
![Table 2. Calculations
~60°C trial
~50°C trial
~40°C trial
~30°C trial
~20°C trial
Vol. of HCI used
(mL)
19.87
12.93
12.37
9.03
6.30
Moles of HCI
used (mol)
9.94
6.47
6.19
4.52
3.15
Moles of borate
present (mol)
[Borate] (M)
Ksp
In(Ksp)
1/T (K-1)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F108a0acc-c86a-4fb9-81b6-4d3403f04067%2F2ebda04f-69a5-46c0-8887-36e9967dfbac%2Fv4wciidg_processed.png&w=3840&q=75)
Transcribed Image Text:Table 2. Calculations
~60°C trial
~50°C trial
~40°C trial
~30°C trial
~20°C trial
Vol. of HCI used
(mL)
19.87
12.93
12.37
9.03
6.30
Moles of HCI
used (mol)
9.94
6.47
6.19
4.52
3.15
Moles of borate
present (mol)
[Borate] (M)
Ksp
In(Ksp)
1/T (K-1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



