UPLOAD Alka Seltzer Repo X General Chemistry 1 Lab M x 9 UPLOAD Density Report: M X What Kind of Reac 671?module_item_id%3D9934003 otx - 5.pptx (48.7 MB) Oxidation-Reduction Reactions Indicate the oxidation state of each element in the following: Mn, K,S, Mn²+, Sg, Mn,O3, SO2, MnO2, SO3, KMNO4, SO,², MnO, , Na,SO,, Na,S,O, Indicate whether or not the following reactions are redox reactions. If they are, identify the species that is (1) oxidized, (2) reduced, (3) the oxidizing agent, (4) the reducing agent. (balance the equations first – write net ionic equations if necessary) a. Mg(s) + H,0(1) → Mg(OH),(aq) + H2(g) b. Al(s) + Sn²*(aq) → Al3*(aq) + Sn(s) c Lifs) CL(a)
UPLOAD Alka Seltzer Repo X General Chemistry 1 Lab M x 9 UPLOAD Density Report: M X What Kind of Reac 671?module_item_id%3D9934003 otx - 5.pptx (48.7 MB) Oxidation-Reduction Reactions Indicate the oxidation state of each element in the following: Mn, K,S, Mn²+, Sg, Mn,O3, SO2, MnO2, SO3, KMNO4, SO,², MnO, , Na,SO,, Na,S,O, Indicate whether or not the following reactions are redox reactions. If they are, identify the species that is (1) oxidized, (2) reduced, (3) the oxidizing agent, (4) the reducing agent. (balance the equations first – write net ionic equations if necessary) a. Mg(s) + H,0(1) → Mg(OH),(aq) + H2(g) b. Al(s) + Sn²*(aq) → Al3*(aq) + Sn(s) c Lifs) CL(a)
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 26Q: In theory, most metals should easily corrode in air. Why? A group of metals called the noble metals...
Related questions
Question
100%
How would you solve these problems? The professor wasn’t able to finish going over this slide in class.
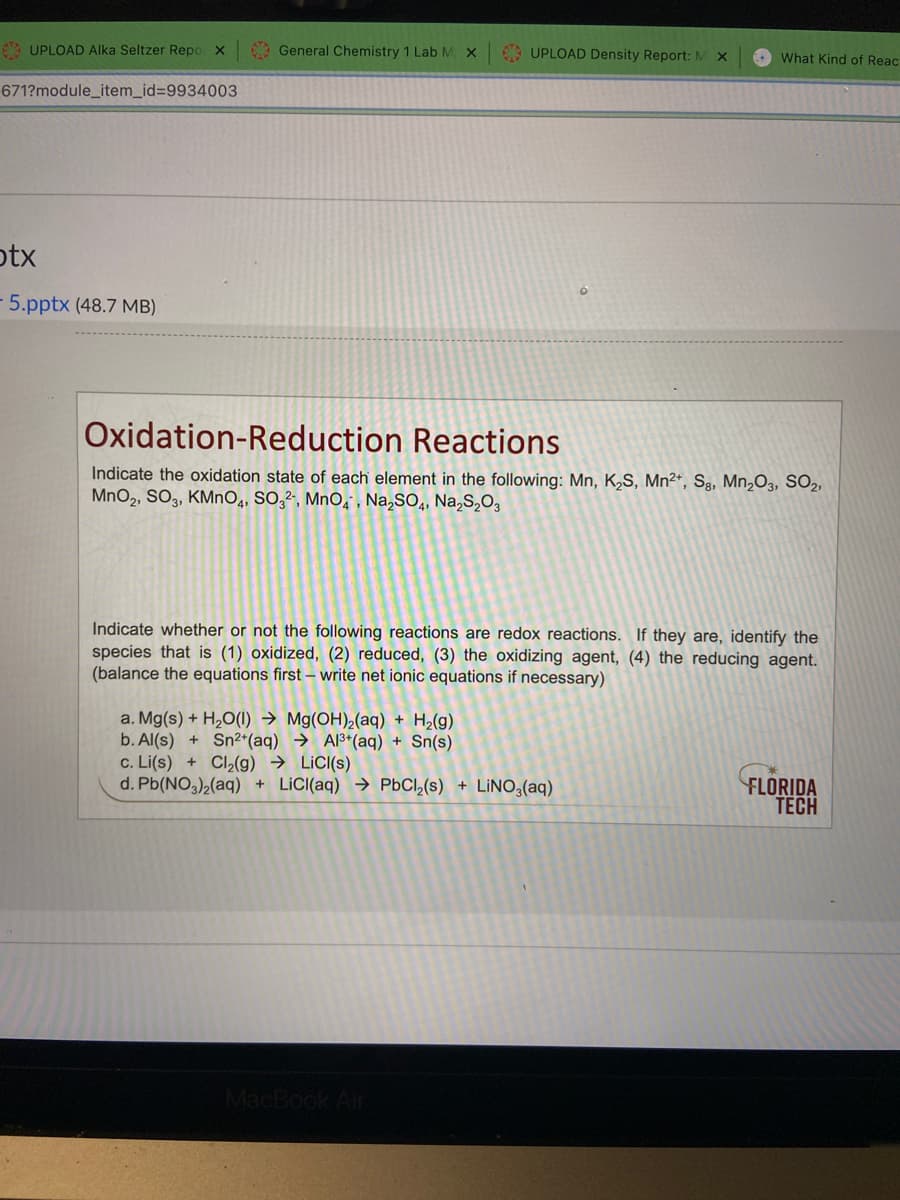
Transcribed Image Text:6 UPLOAD AIka Seltzer Repo x
General Chemistry 1 Lab M X
9 UPLOAD Density Report: M
O What Kind of Reac
671?module_item_id=D9934003
otx
5.pptx (48.7 MB)
Oxidation-Reduction Reactions
Indicate the oxidation state of each element in the following: Mn, K,S, Mn²*, Sg, Mn¿O3, SO2,
MnO2, SO3, KMNO4, SO,², MnO, , Na,SO,, Na,S,O,
Indicate whether or not the following reactions are redox reactions. If they are, identify the
species that is (1) oxidized, (2) reduced, (3) the oxidizing agent, (4) the reducing agent.
(balance the equations first – write net ionic equations if necessary)
a. Mg(s) + H2O(1) → Mg(OH),(aq) + H2(g)
b. Al(s) + Sn²*(aq) → Al3*(aq) + Sn(s)
c. Li(s) + Cl2(g) → LiC((s)
d. Pb(NO,)2(aq) + LICI(aq) → PBCI,(s) + LINO3(aq)
FLORIDA
TECH
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning