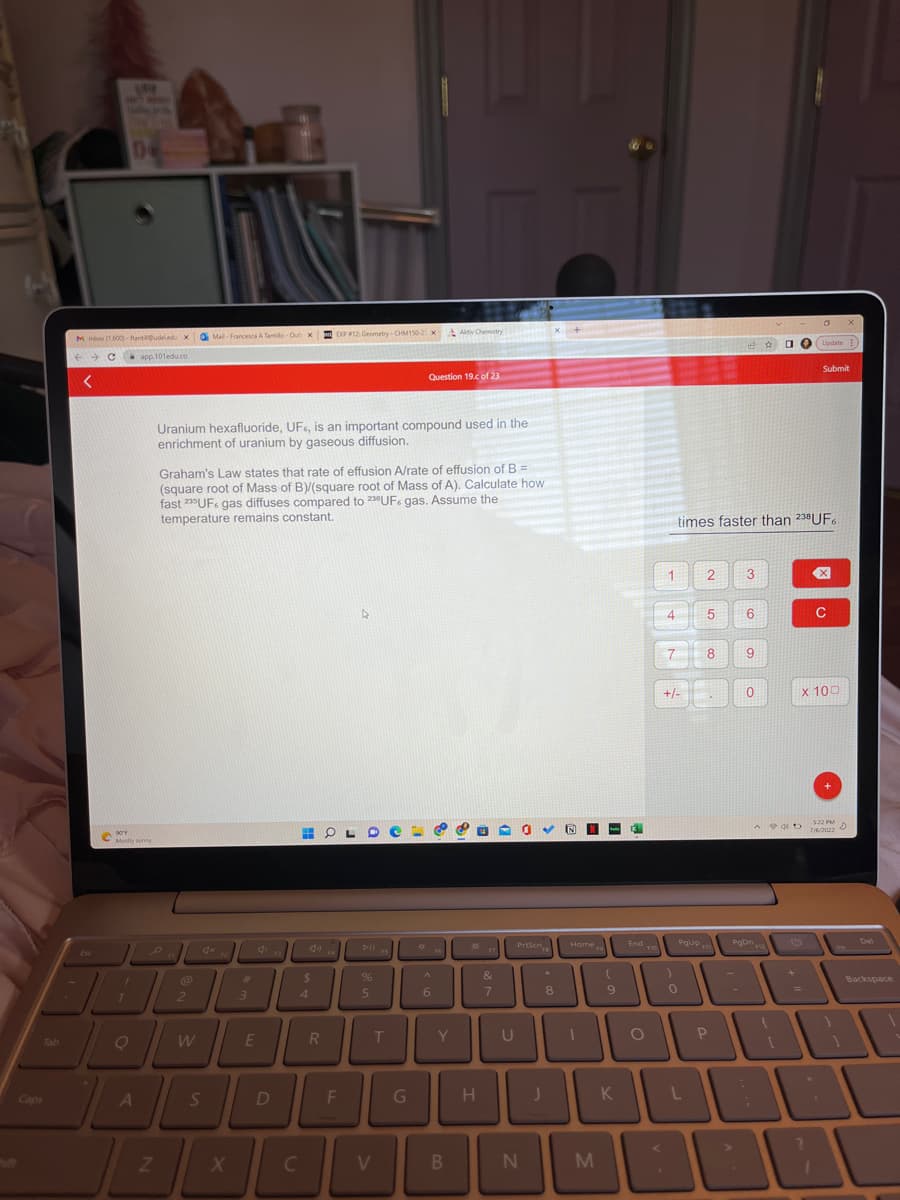
Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. Graham's Law states that rate of effusion A/rate of effusion of B = (square root of Mass of B)/(square root of Mass of A). Calculate how fast 220UF gas diffuses compared to 230UF gas. Assume the temperature remains constant.
Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. Graham's Law states that rate of effusion A/rate of effusion of B = (square root of Mass of B)/(square root of Mass of A). Calculate how fast 220UF gas diffuses compared to 230UF gas. Assume the temperature remains constant.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 93QAP
Related questions
Question
Transcribed Image Text:Caps
Tab
Wall
wen
M Inbox (1.600)-ftantiudeledux Mail-Francesca A Tantillo-Out xEXP #12: Geometry-CHM150-2 x Aktiv Chemistry
← → C
app.101edu.co
90°F
Mostly sunny
1
D
Q
Z
Uranium hexafluoride, UF, is an important compound used in the
enrichment of uranium by gaseous diffusion.
A
A
Graham's Law states that rate of effusion A/rate of effusion of B =
(square root of Mass of B)/(square root of Mass of A). Calculate how
fast 235 UF gas diffuses compared to 230UF. gas. Assume the
temperature remains constant.
2
W
S
3
E
D
#
C
$
4
R
OL
F
C
▷ll s
FS
%
5
T
V
C
Question 19.c of 23
G
P
A
6
Y
B
H
&
7
PrtScn
N
J
8
N
Home
1
M
(
9
K
End o
O
<
1
4
7
+/-
PgUp
0
L
times faster than 238UF
2
5
8
12 4
P
3
6
9
0
□
A PED
PgOn
1924
G
+
0
Update
Submit
C
x 100
BE
5:22 PM
7/6/2022
Del
Backspace
과
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning