Use a table of Standard Reduction Potentials to predict if. If a reaction will occur, write a balanced not ionic F₂(a) when the two are brought in contact via standard half-cells in a voltaic cell. leave all boxes blank... at the productions are in aqueous solution. no reaction will
Use a table of Standard Reduction Potentials to predict if. If a reaction will occur, write a balanced not ionic F₂(a) when the two are brought in contact via standard half-cells in a voltaic cell. leave all boxes blank... at the productions are in aqueous solution. no reaction will
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 31E: Determine the standard cell potential and the cell potential under the stated conditions for the...
Related questions
Question
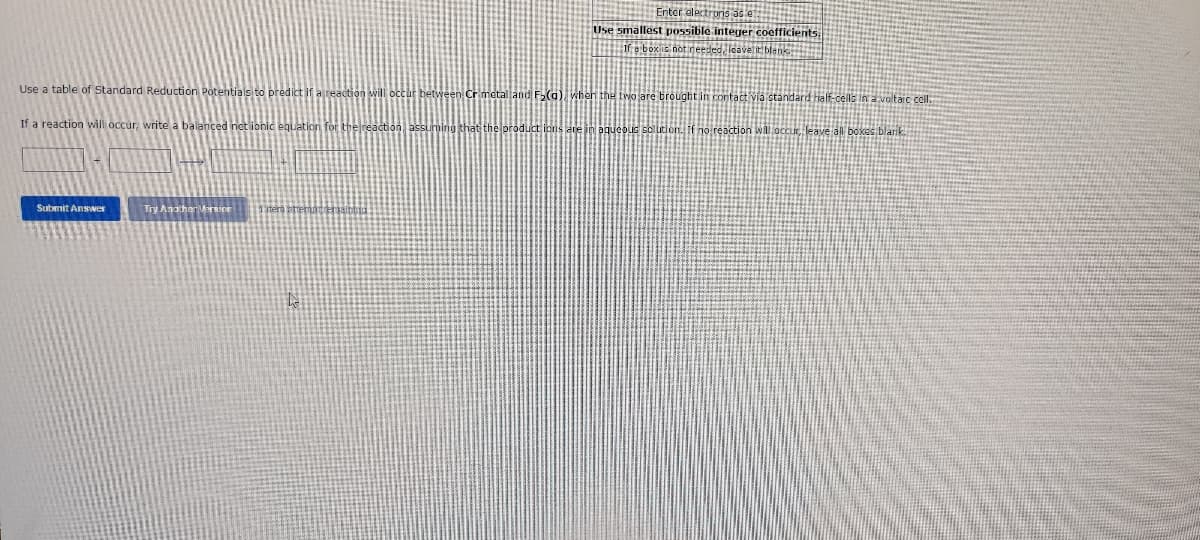
Transcribed Image Text:Use a table of Standard Reduction Potentials to predict if
If a reaction will occur, write a balanced net ionic Jation for
Submit Answer
Try Anoth
that t
Enter electrons as e::
Use smallest possible integer coefficients.
e box is not needed, leaveti
F₂(a), when the two are brought in contact via standard half-cells in a voltaic cell.
aqueous solution. If no reaction will occur, leave all boxes blank.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning