Use periodic trends to compare the elements K, Ge, Si, and S, with regard to the following properties. 17 18 2 H Н Не 2 13 14 15 16 3 4 5 7 8 10 Li Be В C N 0FNE 1 12 13 14 15 16 17 18 Na Mg 3 4 5 6 7 8 9 10 Al Si S CI Ar 11 12 20 23 V 19 21 22 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca| Sc Ti Cr | Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 Rb 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Sr Y Zr | Nb Mo Tc | Ru Rh Pd Ag Cd In Sn Sb Te I Хе 55 57 72 73 74 W 56 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba | La Hf Ta Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 87 88 89 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Ac Rf Db | Sg Bh Hs Mt Ds Rg Cn Nh FI Mc Lv Ts Og 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr|Nd Pm | Sm| Eu Gd Tb | Dy Ho Er Tm Yb Lu 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr a. Which has the largest atomic radius? b. Which has the most negative electron affinity? C. Place the elements in order of increasing ionization energy.
Use periodic trends to compare the elements K, Ge, Si, and S, with regard to the following properties. 17 18 2 H Н Не 2 13 14 15 16 3 4 5 7 8 10 Li Be В C N 0FNE 1 12 13 14 15 16 17 18 Na Mg 3 4 5 6 7 8 9 10 Al Si S CI Ar 11 12 20 23 V 19 21 22 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca| Sc Ti Cr | Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 Rb 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Sr Y Zr | Nb Mo Tc | Ru Rh Pd Ag Cd In Sn Sb Te I Хе 55 57 72 73 74 W 56 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba | La Hf Ta Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 87 88 89 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Ac Rf Db | Sg Bh Hs Mt Ds Rg Cn Nh FI Mc Lv Ts Og 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr|Nd Pm | Sm| Eu Gd Tb | Dy Ho Er Tm Yb Lu 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr a. Which has the largest atomic radius? b. Which has the most negative electron affinity? C. Place the elements in order of increasing ionization energy.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 1P: Before the element scandium was discovered in 1879, it was known as “eka-boron.” Predict the...
Related questions
Question
100%
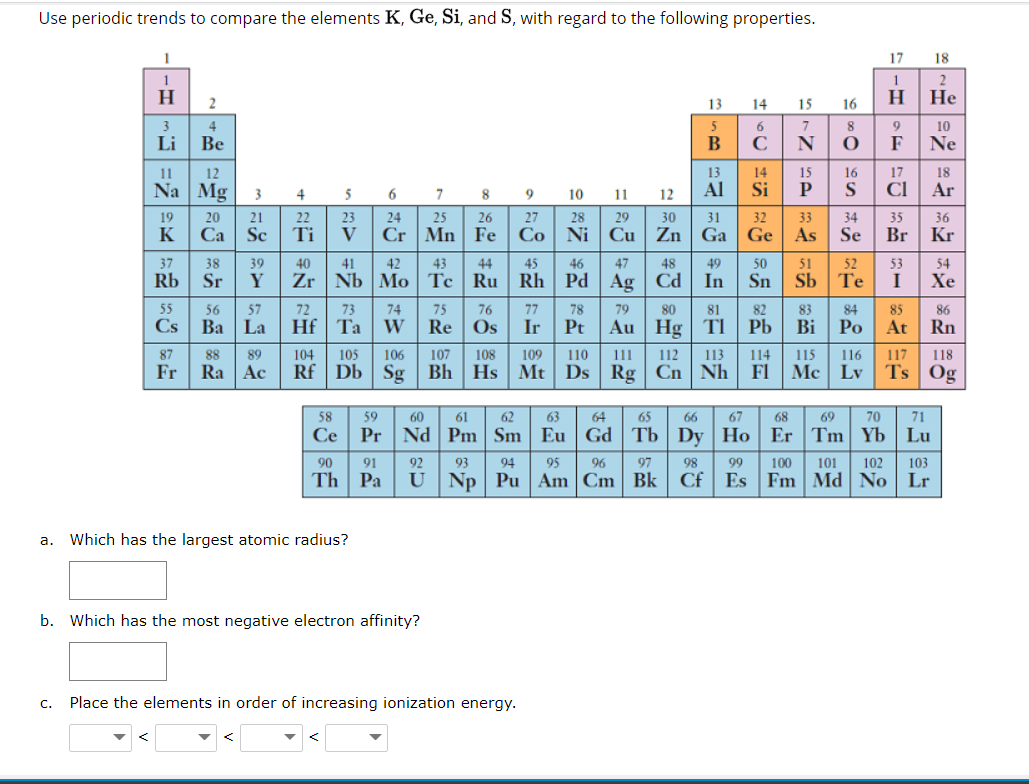
Transcribed Image Text:Use periodic trends to compare the elements K, Ge, Si, and S, with regard to the following properties.
1
17
18
2
H.
H.
Не
2
13
14
15
16
3
4
6.
8
9
10
Li
Be
N
F
Ne
11
12
13
14
15
16
17
18
Na Mg
6
Al
Si
P
S
CI
Ar
3
4
5
7
8
9.
10
11
12
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Са
Sc
Ti
V
Cr
Mn Fe
Co
Ni
Cu
Zn
Ga Ge As
Se
Br
Kr
37
38
39
40
41
42
Mo Tc
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr | Nb
Ru
Rh
Pd
Ag Cd
In
Sn
Sb
Те
I
Хе
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Cs
Ва
La
Hf
Ta
W
Re
Os
Ir
Pt
Au Hg
TI
Pb
Bi
Po
At
Rn
87
88
105
107
112
113
Nh
115
Mc
89
104
106
108
109
110
111
114
116
117
118
Fr
Ra Ac
Rf Db
Sg
Bh Hs
Mt Ds | Rg| Cn
FI
Lv
Ts Og
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Ce Pr Nd Pm Sm| Eu Gd Tb| Dy Ho
Er Tm Yb
Lu
92
93
94
95
97
98
99
Cf Es
100
Th Pa
U Np| Pu Am Cm Bk
Fm Md No
Lr
а.
Which has the largest atomic radius?
b. Which has the most negative electron affinity?
C.
Place the elements in order of increasing ionization energy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning