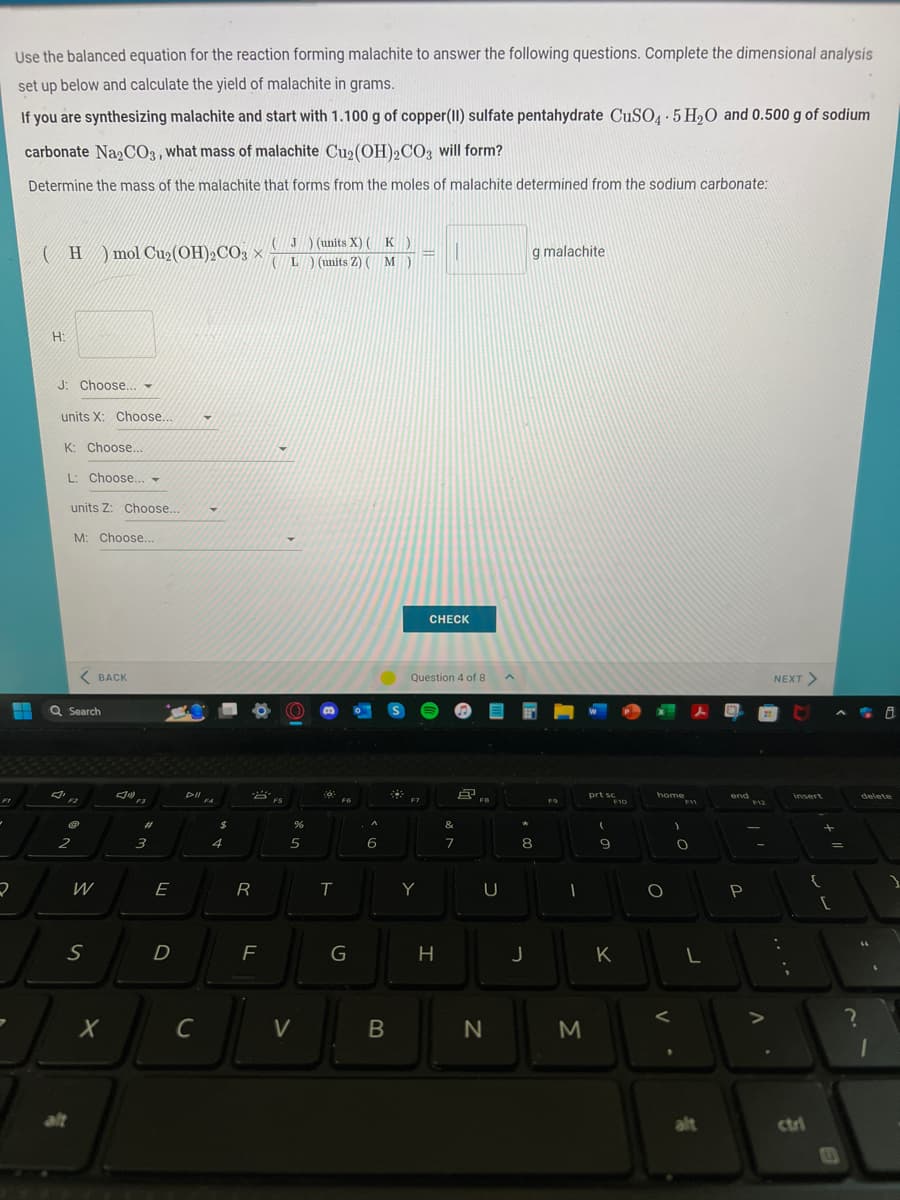
Use the balanced equation for the reaction forming malachite to answer the following questions. Complete the dimensional analysis set up below and calculate the yield of malachite in grams. If you are synthesizing malachite and start with 1.100 g of copper(II) sulfate pentahydrate CuSO4.5H₂O and 0.500 g of sodium carbonate Na2CO3, what mass of malachite Cu₂(OH)2CO3 will form? Determine the mass of the malachite that forms from the moles of malachite determined from the sodium carbonate: (H) mol Cu₂(OH)2CO3 X H: J: Choose... - units X: Choose... K: Choose... L: Choose... ▼ units Z: Choose... M: Choose... Y (J) (units X) (K) (L) (units Z) ( M - g malachite
Use the balanced equation for the reaction forming malachite to answer the following questions. Complete the dimensional analysis set up below and calculate the yield of malachite in grams. If you are synthesizing malachite and start with 1.100 g of copper(II) sulfate pentahydrate CuSO4.5H₂O and 0.500 g of sodium carbonate Na2CO3, what mass of malachite Cu₂(OH)2CO3 will form? Determine the mass of the malachite that forms from the moles of malachite determined from the sodium carbonate: (H) mol Cu₂(OH)2CO3 X H: J: Choose... - units X: Choose... K: Choose... L: Choose... ▼ units Z: Choose... M: Choose... Y (J) (units X) (K) (L) (units Z) ( M - g malachite
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 117E: Bacterial digestion is an economical method of sewage treatment. The reaction is an intermediate...
Related questions
Question
Need help quickly on this practice problem!
Transcribed Image Text:✓
2
Use the balanced equation for the reaction forming malachite to answer the following questions. Complete the dimensional analysis
set up below and calculate the yield of malachite in grams.
If you are synthesizing malachite and start with 1.100 g of copper (II) sulfate pentahydrate CuSO4.5H₂O and 0.500 g of sodium
carbonate Na2CO3, what mass of malachite Cu₂(OH)2CO3 will form?
Determine the mass of the malachite that forms from the moles of malachite determined from the sodium carbonate:
(H) mol Cu₂ (OH)₂CO3J) (units X) (K)
(L) (units Z) ( M M)
H:
J: Choose...
units X: Choose....
K: Choose...
L: Choose...
units Z: Choose...
M: Choose...
31
Q Search
C
2
<BACK
alt
W
S
X
J
F3
#1
3
E
D
DII
с
▾
FA
$
4
R
F
FS
%
5
V
:0
T
F6
G
A
6
B
x
F7
=
Question 4 of 8
Y
CHECK
H
&
7
Å
FB
U
N
*
g malachite
8
J
F9
I
M
prt sc
(
9
F10
K
home
O
)
F11
O
L
alt
end F12
P
-
V
NEXT >
:
T
insert
ctri
{
=
[
E
?
delete
"
(
8
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning