Use the following balanced equation for problems 1-5. Molar masses are given below. 2 As + 3 H2 2 AsH3 + 150. kcal molar masses 74.92 g 2.02 g 77.95 g How much energy is released when 22.22 g of As are consumed ? A. 11.1 kcal B. 21.4 kcal C. 22.2 kcal D. 42.8 kcal Е. 150. kcal
Q: Use the Michaelis-Menten equation to determine the velocity of reaction when: • [S] = 15.0 mM Vmax =…
A: Michaelis-Menten equation is the important equation that deals with enzyme kinetics. The reactants…
Q: All of the following statements are true about the relationships between [S], Km and Vmax EXCEPT: a.…
A: Enzymes are the biocatalyst that increases the rate of biochemical reaction without itself being…
Q: In designing an experiment, the researcher can often choose many different levels of the various…
A: Design of experiments (DOE) is a tool to systematically plan the experiments to be carried out to…
Q: The vitamin content per ounce for three foods is given in the following table.a. Use matrices to…
A: let food A=xfood B = yfood C = z setting up the equations 3x+y+3z= 147x + 5y + 8z = 32x + 3y + 2z =…
Q: Using conversion factors, solve each of the following clinical problems:a. The physician has ordered…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: The Gibbs free energy can be defined as the maximum amount of non-expansion work performed by a…
A:
Q: To estimate drug dosages, doctors use a patient’s body surface area (BSA)(in meters squared) using…
A: Body surface area (BSA) = hm60,where h is the height in centimeters and m is the mass in kilograms.…
Q: 1. Calculate the reaction Kcat for the Control in experiment (1). 2. Draw a velocity versus [S]…
A: Michaelis Menton equation relates velocity of enzymatic reaction with substrate concentration.…
Q: A monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H*(aq) + A¯(aq)…
A: A weak acid is an acid that undergoes partial dissociation and produces proton and a conjugate base.…
Q: in which of the following alternatives is the greatest amount of energy released from the…
A: Hydrocarbons are organic molecules that are essentially made up of carbon and hydrogen atoms, where…
Q: The mathematical expression for the change in free energy of a system is AG AH TAS %3D Which of the…
A:
Q: At 39.9ºC, a solution of ethanol (XetOH = 0.9006, P * etOH = 130.4 Torr) and isooctane (P * iso =…
A: Raoult's law is generally a chemical law that states that the vapor pressure of a solution is basedc…
Q: The average fuel value of fats is 9kcal / g . A 1 oz French fries typically contains 4.5 g of fat.…
A: All living organisms require energy to carry out cellular functions for the growth and repair of the…
Q: 1. Calculate to prepare a 100 g fish feed that includes 45% crude protein (CP), using Fish meal with…
A: Pearson square method is a simple method to calculate contributions of two or more ingredients in a…
Q: Calculate the concentration of an EDTA solution of which 24.22 mL were needed to titrate the…
A: In laboratories, various methods are employed for determining the concentration of a known analyte.…
Q: Write the Michaelis meNten equation and explain each of the terms.
A: Enzymes are biocatalysts that accelerate the rate of chemical reactions taking place inside the…
Q: The enthalpy of combustion of hard coal averages −35 kJ/g, that of gasoline, 1.28 × 105 kJ/gal. How…
A: The enthalpy of combustion (∆Hc) of a substance is defined as the heat energy released when one mole…
Q: Please provide examples of the (Hardy-Weinberg Law P^2 + 2pq + q^2 = 1)
A: There are two equations necessary to solve a Hardy-Weinberg Equilibrium question:?? + ?? = 1??² +…
Q: The mathematical expression for the change in free energy of a system is AG AH- TAS %3D Which of the…
A: Gibbs free energy is the total amount of energy available in the system to do useful work at…
Q: Predict the diffusivity of human serum albumin at 293 K in water as a dilute solution and compare it…
A: Cells are considered as structural and functional units of life. Cells need to exchange nutrients,…
Q: For the analysis of iron-phenanthroline complex, the color of the solution a.) becomes more intense…
A: Iron(II) reacts with three o-phenanthroline to form a stable red-colored complex. Iron(III) doesn't…
Q: The empirical formula of the sugar glucose is C6H12O6. (a) How many moles are there in 270 g of…
A: Glucose is the natural occurring sugar which contains six carbon atom at aldehyde group therefore…
Q: statement regarding kinetic energy is true? sc.7.P.112 A The kinetic energy of an object is A O…
A: Kinetic energy equals the mass of the moving object times the square of that object's speed (v2).
Q: During constant flight, hummingbirds expend about 2.9 kJ/hr, relying on fat oxidation as an energy…
A: The digestion of sugars and fats are the sources of energy in living beings. Glucose is the primary…
Q: This process requires 151 kJ for every 1.00 mol of Fe3O4 reduced. How much energy (in kilojoules) is…
A: A balanced chemical equation is the chemical equation in which the number of each type of atom is…
Q: The mathematical expression for the change in tree energy of a system is AG AH-TAS Which of the…
A: The given free-energy equation is known as the Gibbs-Helmholtz equation. The expression of the…
Q: The normal concentration of Ca2+ in blood is 5.0 mEq>L. How many milligrams of Ca2+ are in 1.00 L…
A: Calcium (Ca2+) is an important element for humans because it is involved in a variety of biochemical…
Q: Tetrodotoxin, found in the puffer fish, has been investigated for use in treatment of moderate to…
A: Tetrodotoxin (TTX) is an extremely poisonous neurotoxin present in the liver and gonads of several…
Q: At the gym, you expend 230 kcal riding the stationary bicycle for 1 h. ▼ Part A How many moles of…
A: ATP is the form of energy for storage and uses at the cellular level. It stores the energy released…
Q: A cart that weighs 15 kg is rolling down a hill with a velocity of 4.2 m/s2. What is the kinetic…
A: solution : A cart that weighs 15 kg, it means Mass= 15 kg velocity= 4.2 m/s2…
Q: Solve this problem
A: Given, The amount of heat absorbed is 26.06 J. The mass of the diamond is 2.10 g. The initial…
Q: (a) How much time is needed to measure the kinetic energy of an electron whose speed is 10.0 m/s…
A: We are answering part a only pls repost for part b
Q: E ion A formula for a cough syrup contains 1/9 gr of codeine phosphate per teaspoonful. How many…
A: 1 teaspoon= 5 ml 1.5 pint = 852 ml cough syrup contains 179 gm of codeine phosphate per…
Q: 2.. Lipid R undergoes combustion: Lipid X(s) + 23 O2(g) ----> 16 CO2(g) + 16 H2O1) AH° = -9682.1…
A: The Gibbs free energy allows us to calculate the overall amount of energy released or absorbed by a…
Q: Calculate the Gibbs free energy change (G) for the following chemical reaction: glutamate + NH3…
A: Introduction Gibbs free energy is a thermodynamic quantity that gives the measure of work done or…
Q: Undergoing moderate activity, an average person will generate about 350 kJ of heat per hour. Using…
A: Palmitic acid : It is the most common saturated fatty acid found in animals, plants and…
Q: The difference in chemical potential of a particular substance between two regions of a system is…
A: Chemical potential of a species is energy that can be absorbed or released due to a change of the…
Q: Regarding the reasoning for the Michaelis-Menten equation to be unsuitable for accurate analysis of…
A: Extrapolation to Vm is inaccurate and therefore Km also cannot be accurately described
Q: This equation models how pure silver (Ag) is extracted from ores of AgK(CN)2 (potassium…
A: Silver is extracted by Mac-Arthur Forest cyanide Process - Step one is the formation of soluble…
Q: The distribution of Na* ions across a typical biological membrane is 10 mmol/dm³ nside the cell, and…
A: The cell has a semipermeable membrane that restricts the movement of solutes, ions, etc. The…
Q: Nutritional biochemists have known for decades that acidicfoods cooked in cast-iron cookware can…
A: In a chemical reaction, the oxidation state is considered the resulting charge that the atom would…
Q: If experimental determinations of Kp at different temperatures for a given equilibrium in the…
A: Kp is the equilibrium constant calculated from partial pressures of reaction equation. It is a…
Q: An average middle-aged person weighing 90 kg (200 lb.) contains 15 % body fat stored in adipose…
A: procedure: Find the bodyweight of persons and yet find the mass of fat From those values Calculate…
Q: Time Mg (s) +2 HCI (aq) MgCl, (aq) + H, (g) The reaction between solid magnesium and hydrochloric…
A: A chemical or a biochemical reaction occurs when the reactant molecules combine or collide together…
Q: Graphically derive the value of KM from a Michaelis-Menten Plot
A: Enzyme is basically biocatalyst that increase the rate of chemical reaction without itself being…
Q: What is the actual yield of iron in moles? What is the theoretical yield of iron in moles? What is…
A: The chemical name of Fe2O3 is Iron(III) oxide is one of the three oxides of iron. The other oxide of…
Q: Predict the trend of entropy in the following scenario: Entropy of vinegar v. salt A Entropy is…
A: Entropy is a thermodynamic quantity representing the degree of disorder or randomness in the system…
Q: How many colchicine tablets, each containing 600 pg, may be prepared from 30 g of colchicine? (A) 50…
A: The administration of drugs is a fundamental nursing skill and knowledge about this important to…
Q: Prozac, C17H18F3NO, is a widely used antidepressant that inhibits the uptake of serotonin by the…
A: Prozac Antidepressant sold as Fluoxetine inhibits the uptake of serotonin by the brain generally…
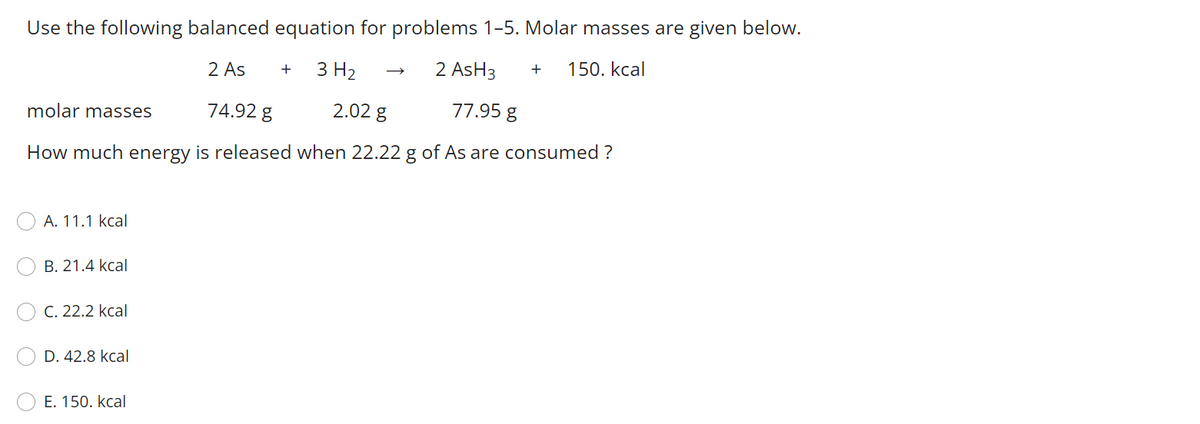
Step by step
Solved in 2 steps

- The following reaction plays a key role in the destruction of ozone in the atmosphere: Cl(g)+ O3 (g)-> ClO(g)+O2 (g) Given the standard molar entropies (S°) below, calculate the ΔS for this reaction. S°C1O = 218.9 J/mol*K S°O3 = 238.8 J/mol*K S°Cl = 165.2 J/mol*K S°O2= 205.0 J/mol*K _______ J/K = ΔSFor the following reaction 3 experiments have been run and the data collected is in the following table @ 35 degrees Celsius 2 NO2F(g) ---> 2 NO2(g) + F2(g) Experiment [NO2F], M Rates, M/s 1 0.263 0.168 2 0.349 0.223 3 0.421 0.269 a) How long will it take for a 65% NO2F solution to become a 31% NO2F solution @35 degrees Celsius?(Hint: Use mass ratios and assume ~1g/ml for density of solutions to get you started) b) It has been determined that at 75 degrees Celsius the rate constant is 1.046 s-1. Calculate the activation energy for the decomposition of NO2F. [Hint: ]ln?1?2=?a?(1?2―1?1) c) What is the half-life of a 35% solution of NO2F @ 35 degrees Celsius?Calculate the Keq (report up to two decimal places and do not use scientific notation) for the net reaction at 298.15K. (see attached image) Note: R = 1.98 x 10 -3 kcal/mol-K
- For ferrocene (C10H10Fe) the enthalpy of sublimation is 73.2 kJ/mol and the entropy of sublimation is 243 J/mol.K. What is the sublimation temperature of ferrocene in degrees Celsius?Calculate ΔG° (answer in kJ/mol) for each of the following reactions from the equilibrium constant at the temperature given. (d)CoO(s)+CO(g)⇌Co(s)+CO2(g) T=550°C Kp=4.90×102 (e)CH3NH2(aq)+H2O(l)⟶CH3NH3+(aq)+OH−(aq) T=25°C Kp=4.4×10−4 (f)PbI2(s)⟶Pb2+(aq)+2I−(aq) T=25°C Kp=8.7×10Regarding the reasoning for the Michaelis-Menten equation to be unsuitable for accurate analysis of experimental data, select all that apply: It is nonlinear It is only valid for reactions at equilibrium It is not valid under experimental conditions Extrapolation to Vm is inaccurate and therefore Km also cannot be accurately described
- Consider the generic reaction2A+3B→2C,ΔHrxn=−100kJIf a reaction mixture initially contains 5 mol of A and 6 mol of B, how much heat (in kJ) will have evolved once the reaction has occurred to the greatest extent possible? 150kj 200kj 300kj 100kJCalculate the overall ΔG° (report up to two decimal places) for the net reaction (see attached image). Answer: _____ kcal/mol Note: R = 1.98 x 10 -3 kcal/mol-KThis process requires 151 kJ for every 1.00 mol of Fe3O4 reduced. How much energy (in kilojoules) is required to produce 55 g of iron?
- The equation of the double reciprocal plot is y = 0.5294 x + 1.4960. What is the value of vmax (in M/s)? The substrate concentration is given in units of molarity (M) and reaction velocity has units of molarity per second (M/s). (Report to three significant figures)Combining 0.304 mol Fe2O30.304 mol Fe2O3 with excess carbon produced 11.6 g Fe.11.6 g Fe. Fe2O3+3C⟶2Fe+3COFe2O3+3C⟶2Fe+3CO What is the actual yield of iron in moles? What is the theoretical yield of iron in moles? What is the percent yield?The enthalpy of combustion of hard coal averages −35 kJ/g, that of gasoline, 1.28 × 105 kJ/gal. How many kilograms of hard coal provide the same amount of heat as is available from 1.0 gallon of gasoline? Assume that thedensity of gasoline is 0.692 g/mL (the same as the density of isooctane).



