Use the following information to answer question 36. Trichloroacetic acid, CC13COOH(aq), is used in cosmetic treatments to improve the skin's The Brønsted-Lowry equation for the reaction of CCI,COOH(aq) with water is appearance. shown below. CC13COOH(aq) + H₂O(1) CCl₂COO (aq) + H₂O*(aq) 36. Which of the following rows identifies a conjugate acid-base pair in the reaction and a species that can be classified as amphiprotic? Row Conjugate Acid-Base Pair CC13COOH(aq)/H3O+ (aq) A. B. CC13COOH(aq)/H3O+ (aq) CC13COOH(aq)/CC13COO (aq) C. D. CCI3COOH(aq)/CC13COO (aq) Use the following information to answer question 37. A group of students was instructed to make a buffer solution. The group proposed three different mixtures. I Amphiprotic Species H₂O(1) H3O+ (aq) H₂O(1) or to doirt W BE H3O+ (aq) II Equal volumes of 0.10 mol/L HNO₂(aq) and 0.10 mol/L NaOH(aq) Equal volumes of 0.10 mol/L H₂SO3(aq) and 0.10 mol/L NaHSO3(aq)
Use the following information to answer question 36. Trichloroacetic acid, CC13COOH(aq), is used in cosmetic treatments to improve the skin's The Brønsted-Lowry equation for the reaction of CCI,COOH(aq) with water is appearance. shown below. CC13COOH(aq) + H₂O(1) CCl₂COO (aq) + H₂O*(aq) 36. Which of the following rows identifies a conjugate acid-base pair in the reaction and a species that can be classified as amphiprotic? Row Conjugate Acid-Base Pair CC13COOH(aq)/H3O+ (aq) A. B. CC13COOH(aq)/H3O+ (aq) CC13COOH(aq)/CC13COO (aq) C. D. CCI3COOH(aq)/CC13COO (aq) Use the following information to answer question 37. A group of students was instructed to make a buffer solution. The group proposed three different mixtures. I Amphiprotic Species H₂O(1) H3O+ (aq) H₂O(1) or to doirt W BE H3O+ (aq) II Equal volumes of 0.10 mol/L HNO₂(aq) and 0.10 mol/L NaOH(aq) Equal volumes of 0.10 mol/L H₂SO3(aq) and 0.10 mol/L NaHSO3(aq)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 64QAP
Related questions
Question
Need both plz
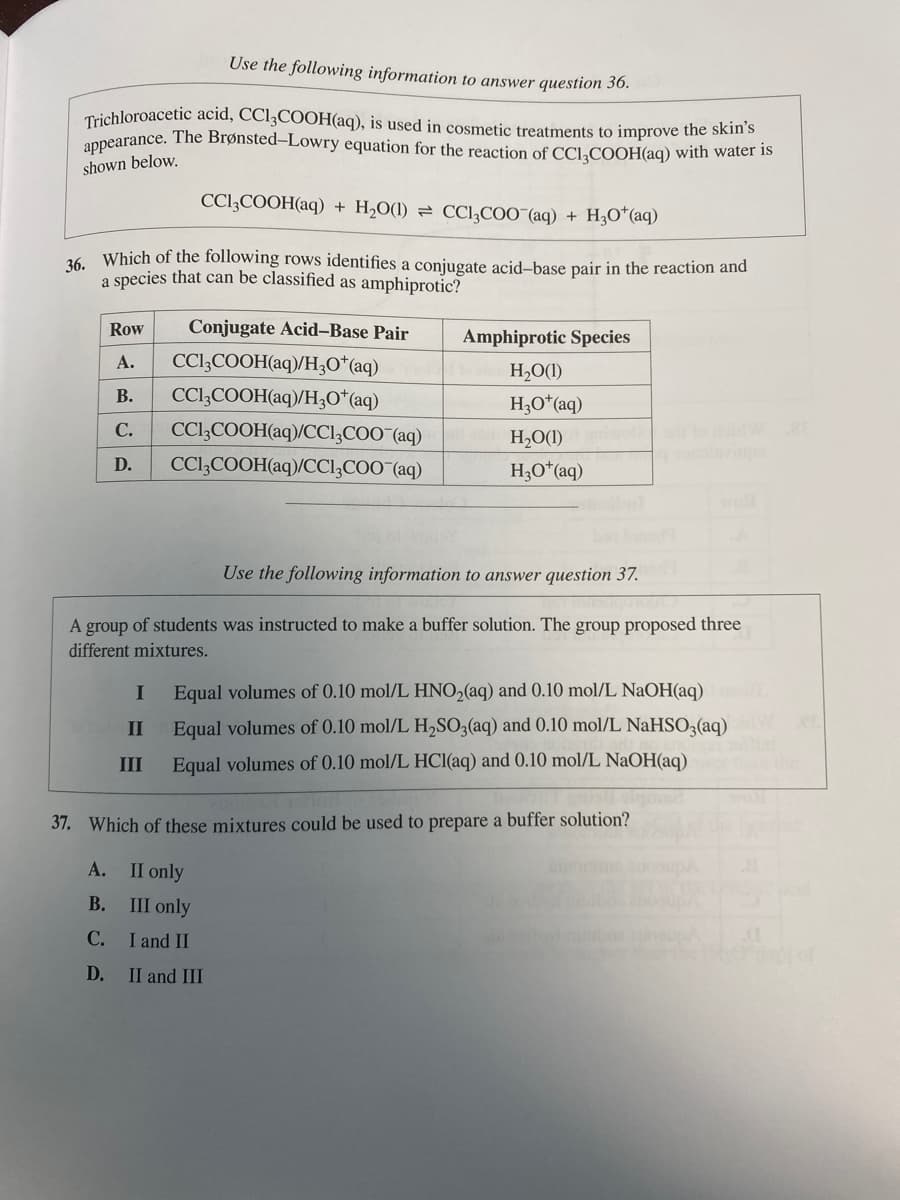
Transcribed Image Text:Use the following information to answer question 36.
Trichloroacetic acid, CC13COOH(aq), is used in cosmetic treatments to improve the skin's
appearance. The Brønsted-Lowry equation for the reaction of CC13COOH(aq) with water is
shown below.
CC13COOH(aq) + H₂O(1) CCl₂COO (aq) + H₂O*(aq)
36. Which of the following rows identifies a conjugate acid-base pair in the reaction and
a species that can be classified as amphiprotic?
Row
Conjugate Acid-Base Pair
CC13COOH(aq)/H3O+ (aq)
A.
B. CC13COOH(aq)/H3O+ (aq)
C. CC13COOH(aq)/CC13COO (aq)
CC13COOH(aq)/CC13COO¯(aq)
D.
Amphiprotic Species
H₂O(1)
H3O+ (aq)
H₂O(1) woli od to doid W BE
H3O+ (aq)
Use the following information to answer question 37.
A group of students was instructed to make a buffer solution. The group proposed three
different mixtures.
I Equal volumes of 0.10 mol/L HNO₂(aq) and 0.10 mol/L NaOH(aq)
II
III
Equal volumes of 0.10 mol/L H₂SO3(aq) and 0.10 mol/L NaHSO3(aq)
Equal volumes of 0.10 mol/L HCl(aq) and 0.10 mol/L NaOH(aq)
37. Which of these mixtures could be used to prepare a buffer solution?
A.
II only
B.
III only
C.
I and II
D.
II and III
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax