Use the ideal gas law to complete the table: Р V n T 2.35 atm 1.28 L 207 K 518 torr 0.746 mol 298 K 0.433 atm 0.183 L |1.25x10-2 mol 22.0 mL 5.75x10-3 mol 20.3°C
Use the ideal gas law to complete the table: Р V n T 2.35 atm 1.28 L 207 K 518 torr 0.746 mol 298 K 0.433 atm 0.183 L |1.25x10-2 mol 22.0 mL 5.75x10-3 mol 20.3°C
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section10.7: Diffusion And Effusion
Problem 1RC: In Figure 10.17, ammonia gas and hydrogen chloride are introduced from opposite ends of a glass...
Related questions
Question
A). Complete the first column of the table?
B). Complete the second column of the table?
C). Complete the third column of the table?
D). Complete the fourth column of the table?
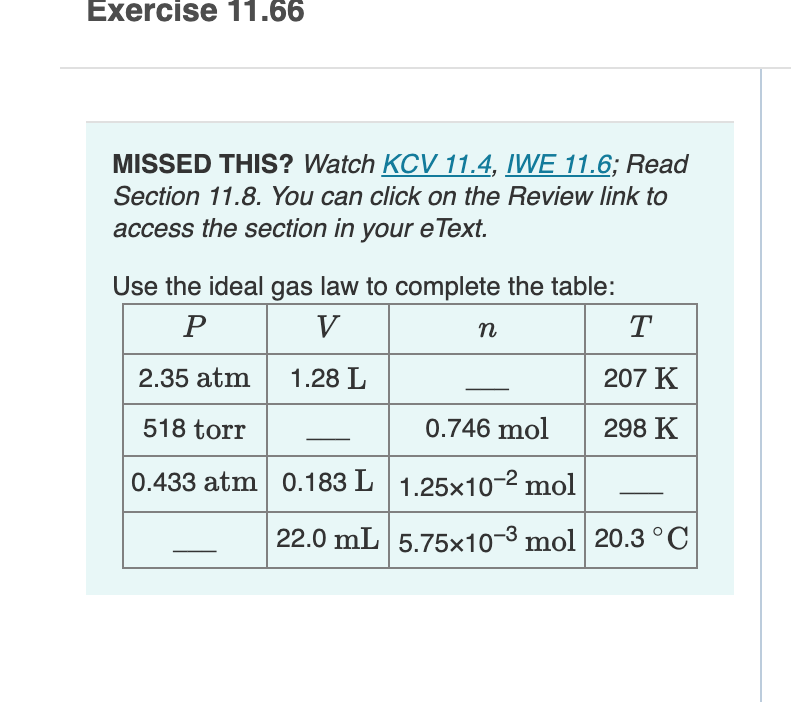
Transcribed Image Text:Exercise 11.66
MISSED THIS? Watch KCV 11.4, IWE 11.6; Read
Section 11.8. You can click on the Review link to
access the section in your eText.
Use the ideal gas law to complete the table:
V
n
T
2.35 atm
1.28 L
207 K
518 torr
0.746 mol
298 K
0.433 atm 0.183 L 1.25x10-2 mol
22.0 mL 5.75x10-3 mol 20.3 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning