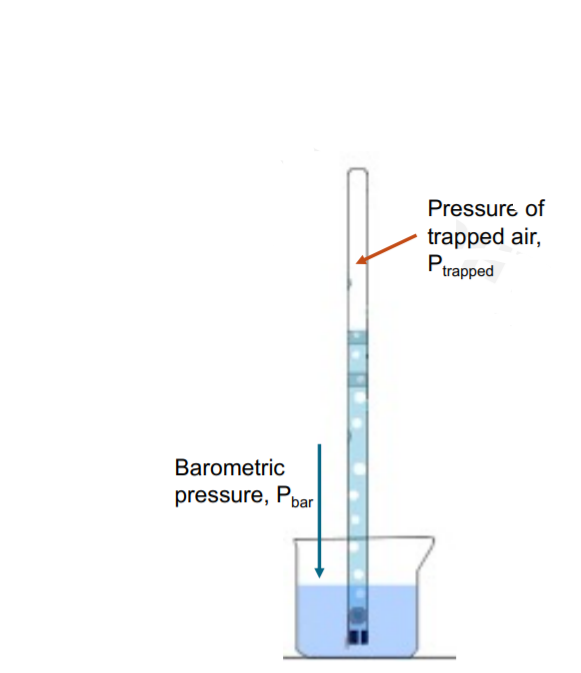
In Dalton's Law of Partial Pressures: Ptrapped gas = Pdry gas + Pwater vapor Why must the pressure of water vapor be included in this calculation? a. The pressure exerted by the evolved H2(g) is reduced because of the co-presence of water vapor, thus Pwater vapor has to be added to Pdry gas. b. Some of the liquid water evaporates into the gas, and increases the total pressure generated by the trapped gas. c. As the H2(g) is bubbled up the reaction solution, some of the H2(g) molecules are solubilized by water in the aqueous solution, effectively decreasing the measured pressure above. d. The pressure contributed by the H2(g) is greater than the measured total pressure because of the contribution of partial pressure of water vapor.
In Dalton's Law of Partial Pressures:
Ptrapped gas = Pdry gas + Pwater vapor
Why must the pressure of water vapor be included in this calculation?
a. The pressure exerted by the evolved H2(g) is reduced because of the co-presence of water vapor, thus Pwater vapor has to be added to Pdry gas.
b. Some of the liquid water evaporates into the gas, and increases the total pressure generated by the trapped gas.
c. As the H2(g) is bubbled up the reaction solution, some of the H2(g) molecules are solubilized by water in the aqueous solution, effectively decreasing the measured pressure above.
d. The pressure contributed by the H2(g) is greater than the measured total pressure because of the contribution of partial pressure of water vapor.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









