Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 16QAP: Computers are not supposed to be in very warm rooms. The highest temperature tolerated for maximum...
Related questions
Question
You can’t excess the interactive so I write down what information was being presented.

Transcribed Image Text:Mossof mustay melel
S.40009
Massof wa tat
25.00g
Noitiel temp cf mustery
25し
Initial tempo@lsoto
28°C
Temputer ms
was burne
66.29
plerad
Tempof wa tar t burnad
25780L
Massof
usatert buenced
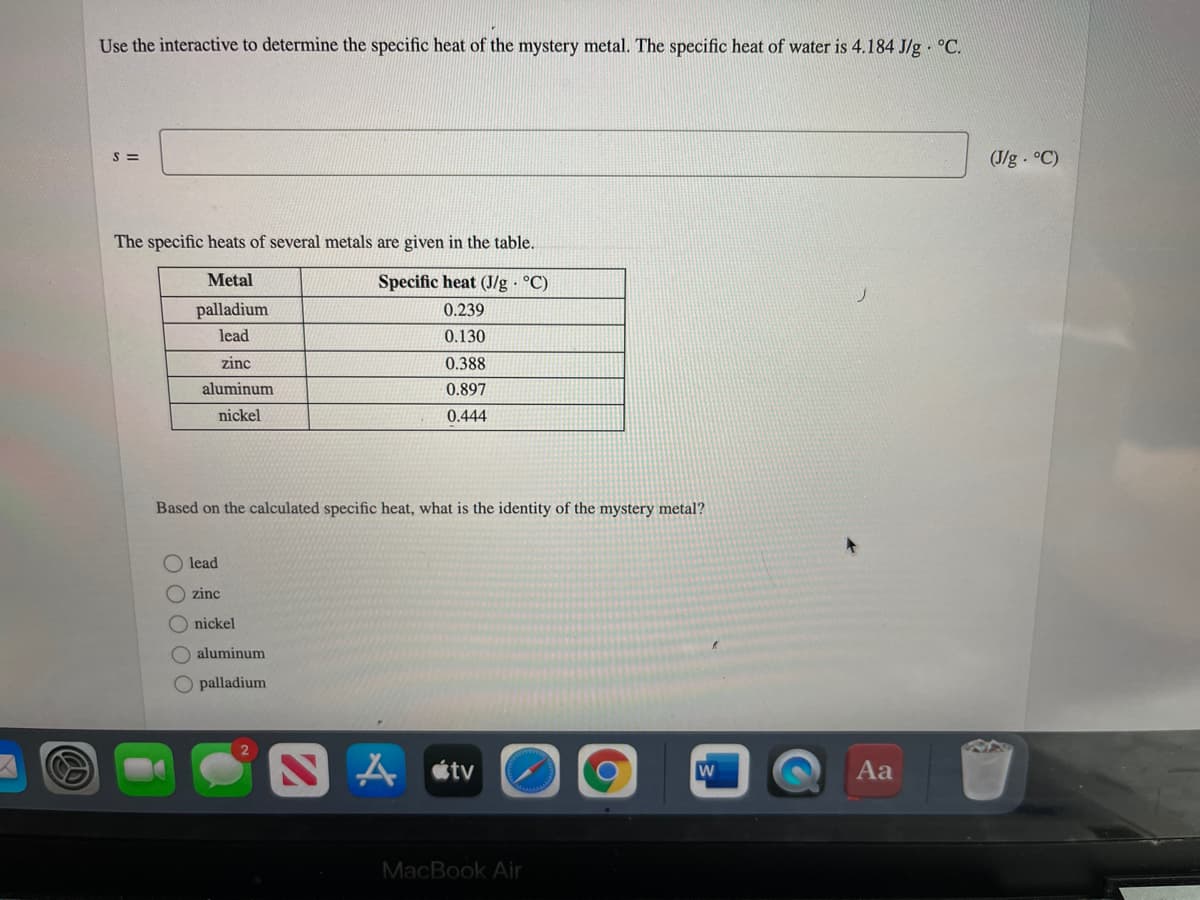
Transcribed Image Text:Use the interactive to determine the specific heat of the mystery metal. The specific heat of water is 4.184 J/g · °C.
S =
(J/g °C)
The specific heats of several metals are given in the table.
Metal
Specific heat (J/g °C)
palladium
0.239
lead
0.130
zinc
0.388
aluminum
0.897
nickel
0.444
Based on the calculated specific heat, what is the identity of the mystery metal?
lead
O zinc
O nickel
O aluminum
O palladium
SA «tv
Aa
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
