Use the observation in the first column to answer the question in the second column. observation question At any temperature where both substances are liquid, which has the higher vapor pressure? Substance C The enthalpy of vaporization of Substance C is smaller than that of Substance D. Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? Substance A At 68 °C, Substance A has a vapor pressure of 114. torr and Substance B has a vapor pressure of 124. torr. Substance B Neither, A and B have the same boiling point. It's impossible to know without more information. Which has a higher vapor pressure? At 1 atm pressure, Substance E Substance E boils at 53. °C and Substance F boils at Substance F 22. °C. Neither, E and F have the same vapor pressure. It's impossible to know without more information. X S ?
Use the observation in the first column to answer the question in the second column. observation question At any temperature where both substances are liquid, which has the higher vapor pressure? Substance C The enthalpy of vaporization of Substance C is smaller than that of Substance D. Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? Substance A At 68 °C, Substance A has a vapor pressure of 114. torr and Substance B has a vapor pressure of 124. torr. Substance B Neither, A and B have the same boiling point. It's impossible to know without more information. Which has a higher vapor pressure? At 1 atm pressure, Substance E Substance E boils at 53. °C and Substance F boils at Substance F 22. °C. Neither, E and F have the same vapor pressure. It's impossible to know without more information. X S ?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 38GQ: The following data are the equilibrium vapor pressure of limonene, C10H16, at various temperatures....
Related questions
Question
Please help me...
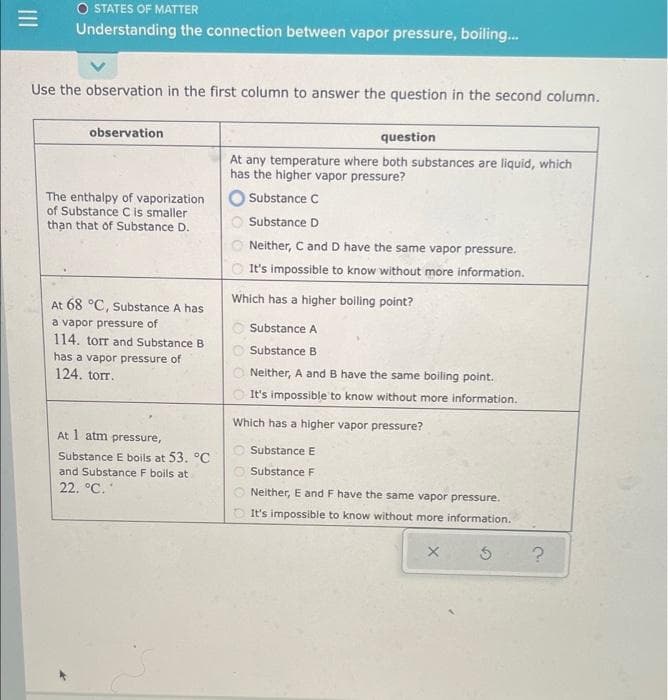
Transcribed Image Text:O STATES OF MATTER
Understanding the connection between vapor pressure, boiling.
Use the observation in the first column to answer the question in the second column.
observation
question
At any temperature where both substances are liquid, which
has the higher vapor pressure?
Substance C
The enthalpy of vaporization
of Substance C is smaller
than that of Substance D.
Substance D
Neither, C and D have the same vapor pressure.
It's impossible to know without more information.
Which has a higher boiling point?
At 68 °C, Substance A has
a vapor pressure of
114. torr and Substance B
Substance A
Substance B
has a vapor pressure of
124. torr.
Neither, A and B have the same boiling point.
It's impossible to know without more information.
Which has a higher vapor pressure?
At 1 atm pressure,
O Substance E
Substance E boils at 53. °C
and Substance F boils at
O Substance F
22. °C.
Neither, E and F have the same vapor pressure.
O It's impossible to know without more information.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning