Use the References to access important values if needed for this question. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. When aqueous solutions of magnesium iodide and ammonium carbonate are combined, solid magnesium carbonate nd a solution of ammonium iodide are formed. The net ionic equation for this reaction is: Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.)
Use the References to access important values if needed for this question. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. When aqueous solutions of magnesium iodide and ammonium carbonate are combined, solid magnesium carbonate nd a solution of ammonium iodide are formed. The net ionic equation for this reaction is: Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 18QAP: On the basis of the general solubility rules given in Table 7.1, write a balanced molecular equation...
Related questions
Question
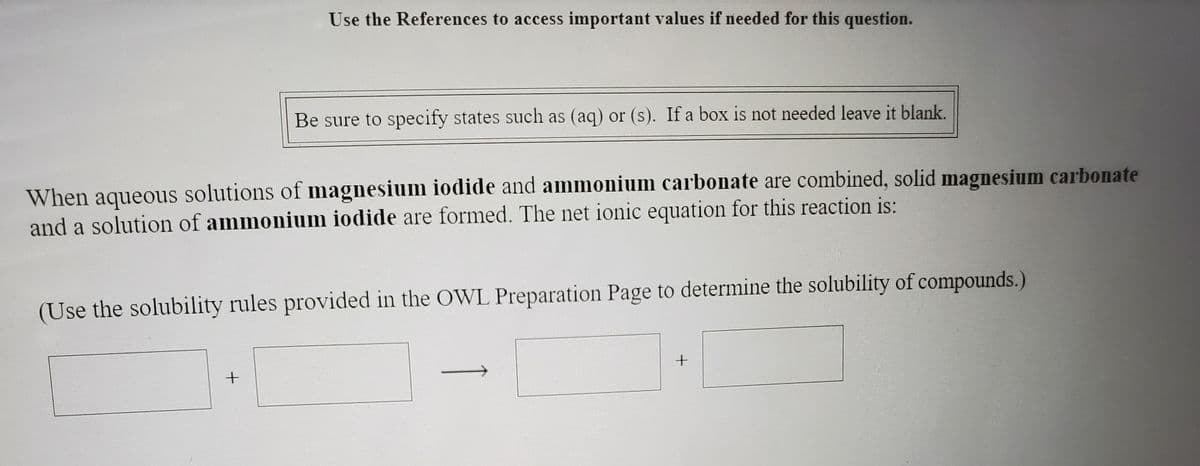
Transcribed Image Text:Use the References to access important values if needed for this question.
Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
When aqueous solutions of magnesium iodide and ammonium carbonate are combined, solid magnesium carbonate
and a solution of ammonium iodide are formed. The net ionic equation for this reaction is:
(Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning