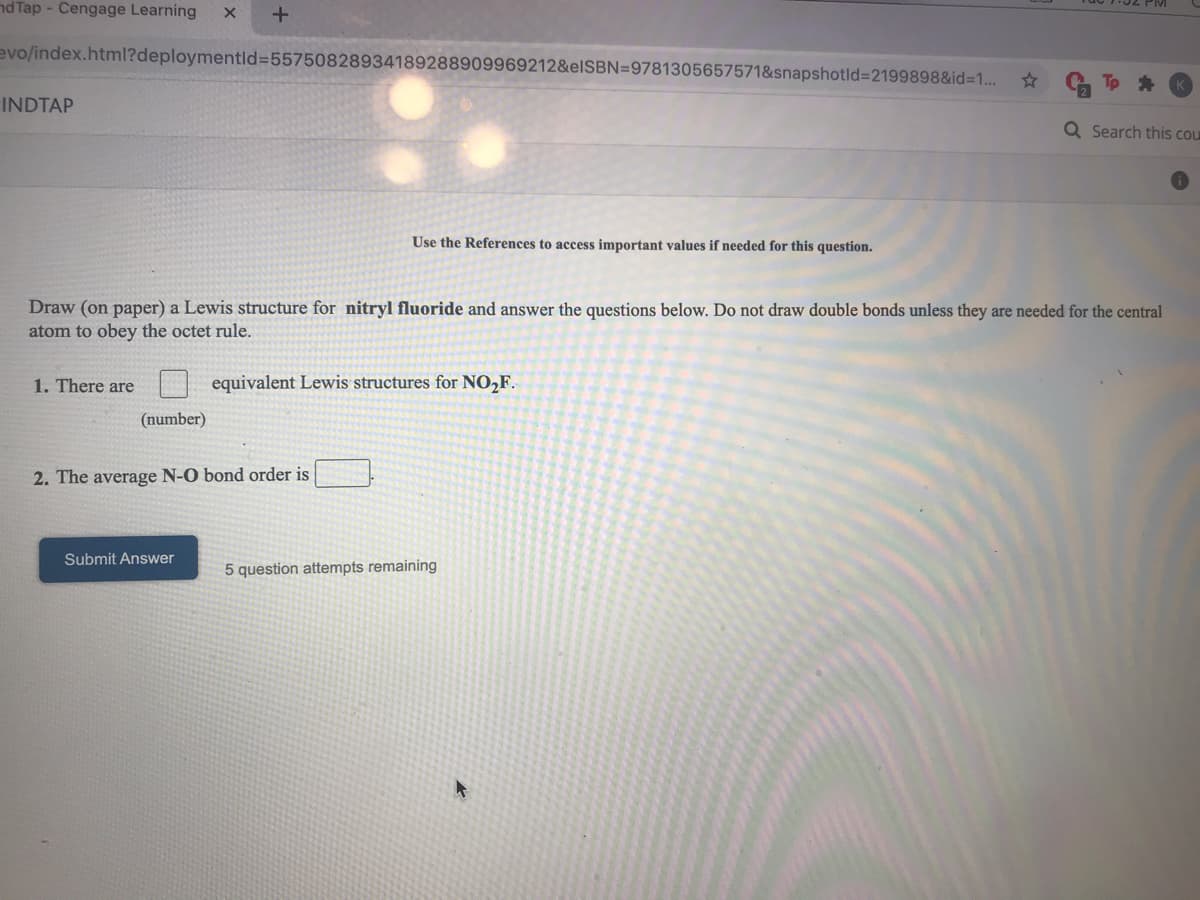
Use the References to access important values if needed for this question. Draw (on paper) a Lewis structure for nitryl fluoride and answer the questions below. Do not draw double bonds unless they are needed for the central atom to obey the octet rule. 1. There are equivalent Lewis structures for NO,F. (number) 2. The average N-O bond order is Submit Answer 5 question attempts remaining
Use the References to access important values if needed for this question. Draw (on paper) a Lewis structure for nitryl fluoride and answer the questions below. Do not draw double bonds unless they are needed for the central atom to obey the octet rule. 1. There are equivalent Lewis structures for NO,F. (number) 2. The average N-O bond order is Submit Answer 5 question attempts remaining
Chapter27: Molecular Fluorescence Spectroscopy
Section: Chapter Questions
Problem 27.11QAP
Related questions
Question
Transcribed Image Text:nd Tap - Cengage Learning
+
evo/index.html?deploymentid%3D55750828934189288909969212&elSBN=9781305657571&snapshotld%32199898&id3D.
TP *
INDTAP
Q Search this cou
Use the References to access important values if needed for this question.
Draw (on paper) a Lewis structure for nitryl fluoride and answer the questions below. Do not draw double bonds unless they are needed for the central
atom to obey the octet rule.
1. There are
equivalent Lewis structures for NO,F.
(number)
2. The average N-O bond order is
Submit Answer
5 question attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



