Use the References to access important values if needed for this question. The freezing point of water is 0.00°C at 1 atmosphere. How many grams of aluminum sulfate (342.2 g/mol), must be dissolved in 275.0 grams of water to reduce the freezing point by 0.350°C ? Refer to the table for the necessary boiling or freezing point constant. Solvent Formula KB CC/m) Kr(°C/m) Water H20 0.512 1.86 Ethanol CH;CH2OH 1.22 1.99 Chloroform CHCI; 3.67 Benzene CH6 2.53 5.12 Diethyl ether CH3CH,OCH,CH3 2.02 g aluminum sulfate. Submit Answer Retry Entire Group 8 more group attempts remaining
Use the References to access important values if needed for this question. The freezing point of water is 0.00°C at 1 atmosphere. How many grams of aluminum sulfate (342.2 g/mol), must be dissolved in 275.0 grams of water to reduce the freezing point by 0.350°C ? Refer to the table for the necessary boiling or freezing point constant. Solvent Formula KB CC/m) Kr(°C/m) Water H20 0.512 1.86 Ethanol CH;CH2OH 1.22 1.99 Chloroform CHCI; 3.67 Benzene CH6 2.53 5.12 Diethyl ether CH3CH,OCH,CH3 2.02 g aluminum sulfate. Submit Answer Retry Entire Group 8 more group attempts remaining
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.98QE
Related questions
Question
I don’t know how to do this question
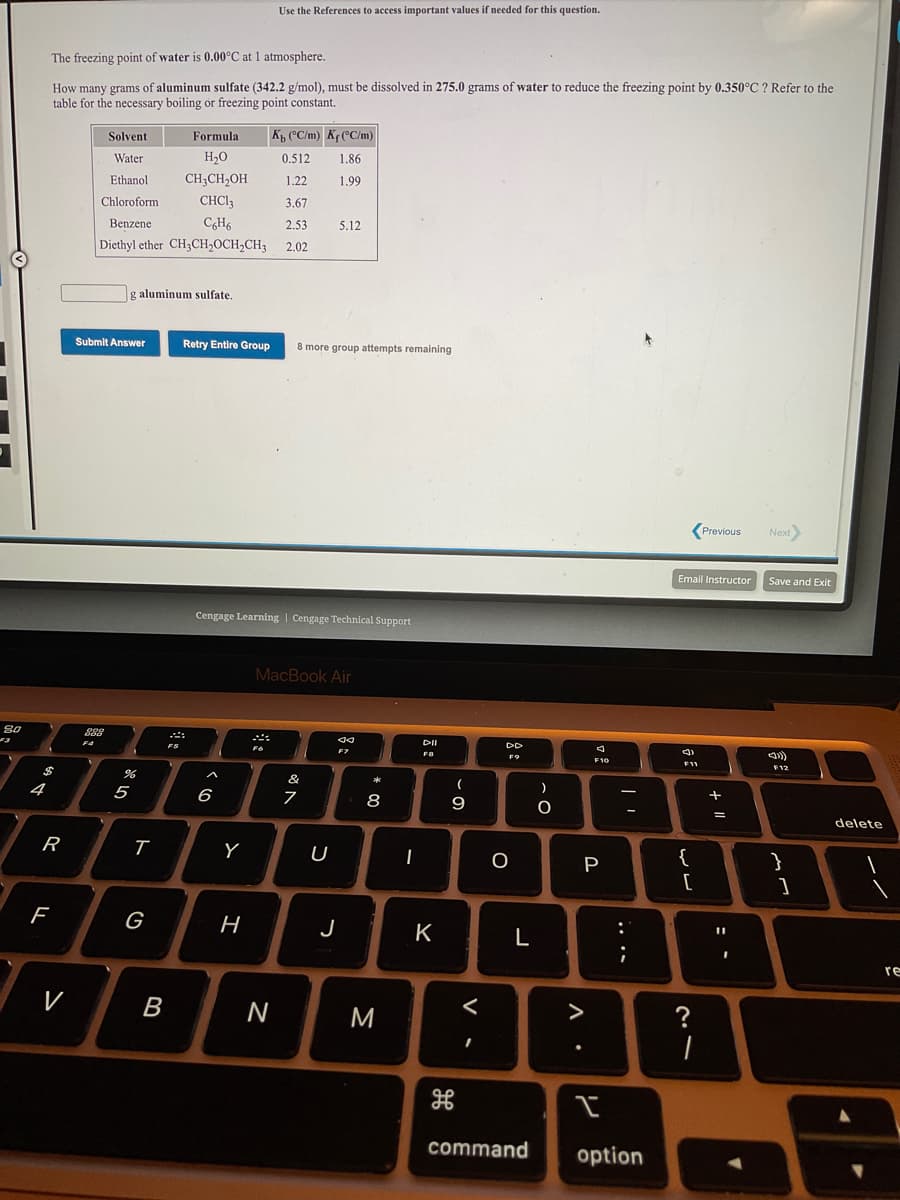
Transcribed Image Text:Use the References to access important values if needed for this question.
The freezing point of water is 0.00°C at 1 atmosphere.
How many grams of aluminum sulfate (342.2 g/mol), must be dissolved in 275.0 grams of water to reduce the freezing point by 0.350°C ? Refer to the
table for the necessary boiling or freezing point constant.
Solvent
Formula
K, (°C/m) Kf (°C/m)
Water
H20
0.512
1.86
Ethanol
CH;CH,OH
1.22
1.99
Chloroform
CHCI3
3.67
Benzene
C6H6
2,53
5.12
Diethyl ether CH3CH,OCH,CH;
2,02
g aluminum sulfate
Submit Answer
Retry Entire Group
8 more group attempts remaining
(Previous
Next
Email Instructor
Save and Exit
Cengage Learning | Cengage Technical Support
MacBook Air
888
こ。
DII
DD
Fア
FO
F10
F11
F12
&
4
5
(
)
6
8
9
%3D
delete
R
U
P
{
}
[
[
G
H
J
K
L
:
%3D
re
M
く
>
?
command
option
ピ
* 00
ト
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning