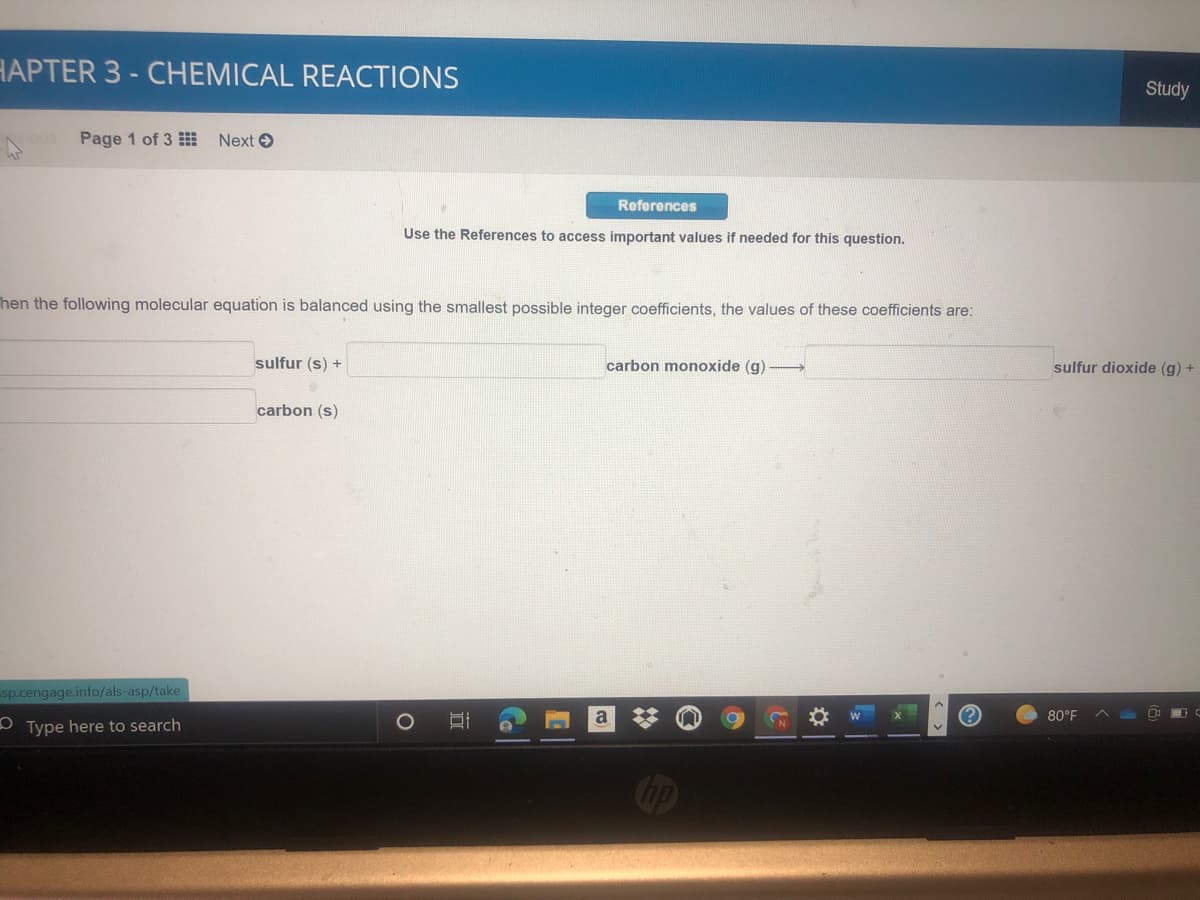
Use the References to access important values if needed for this question. Then the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: sulfur (s) + carbon monoxide (g) sulfur dioxide (g) + carbon (s)
Use the References to access important values if needed for this question. Then the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: sulfur (s) + carbon monoxide (g) sulfur dioxide (g) + carbon (s)
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.3QAP
Related questions
Question
Transcribed Image Text:HAPTER 3 - CHEMICAL REACTIONS
Study
Page 1 of 3
Next O
References
Use the References to access important values if needed for this question.
hen the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are:
sulfur (s) +
carbon monoxide (g)
sulfur dioxide (g) +
carbon (s)
sp.cengage.info/als-asp/take
a
80°F
O Type here to search
Ca
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning